
Prevention and Management of Wasting and Nutritional Edema (Acute Malnutrition) in Infants and Children Under 5 Years
"last update: 28 April 2024"
- Implementation Tools
Educational materials based on this Adapted CPG for treatment of CAP in children have been made available in several forms including:
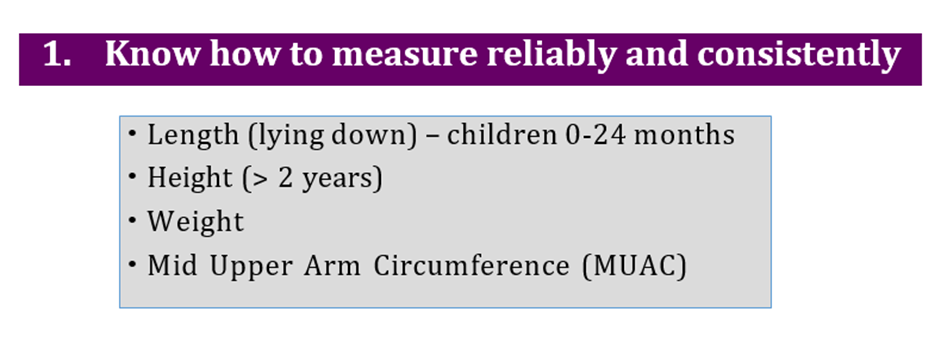
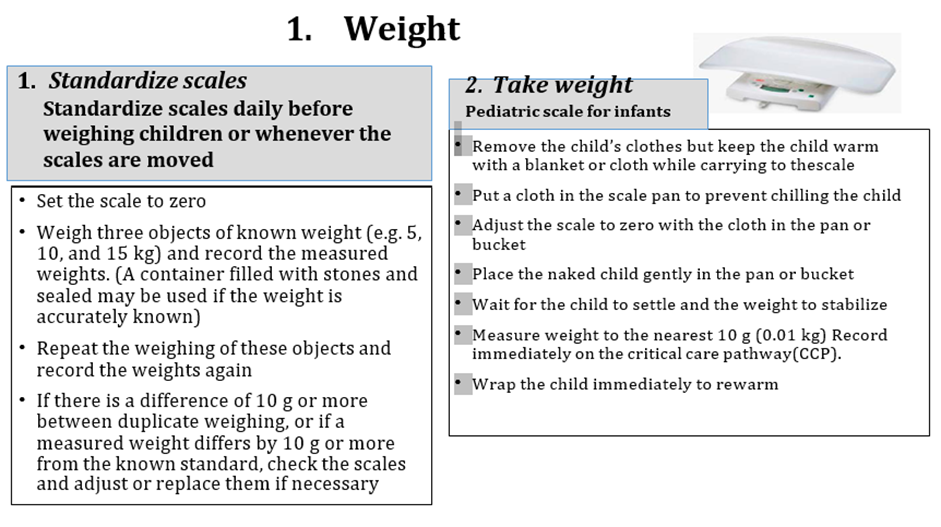
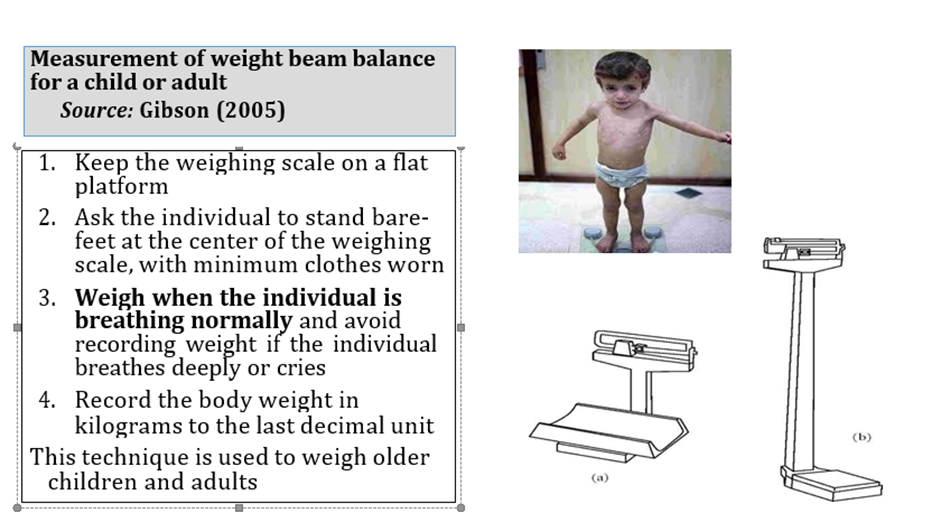
1. Manual for physician for diagnosis and algorithm for management of acute malnutrition
3. Arabic Educational materials for nurses and mothers
















|
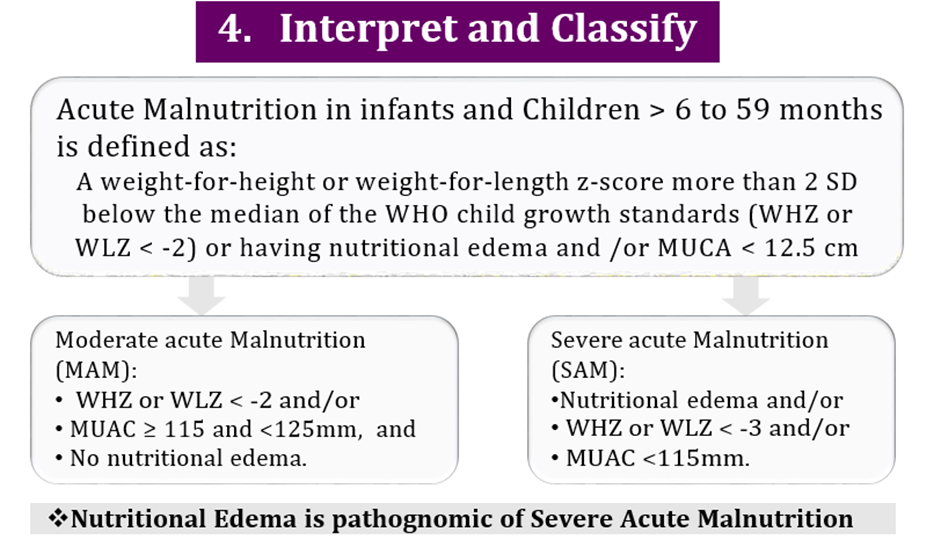
Initiative Nutritional Assessment Date of Visit: Date of Birth or age in months Name: Sex: Weight: gm Height cm Weight/Height ZSMUAC cm Edema: + ++ +++ Interpretation: SAM MAM mild malnutrition |
|
||
|
Clinical Assessment Yes/No |
Dietary Assessment |
Laboratory Assessment |
|
|
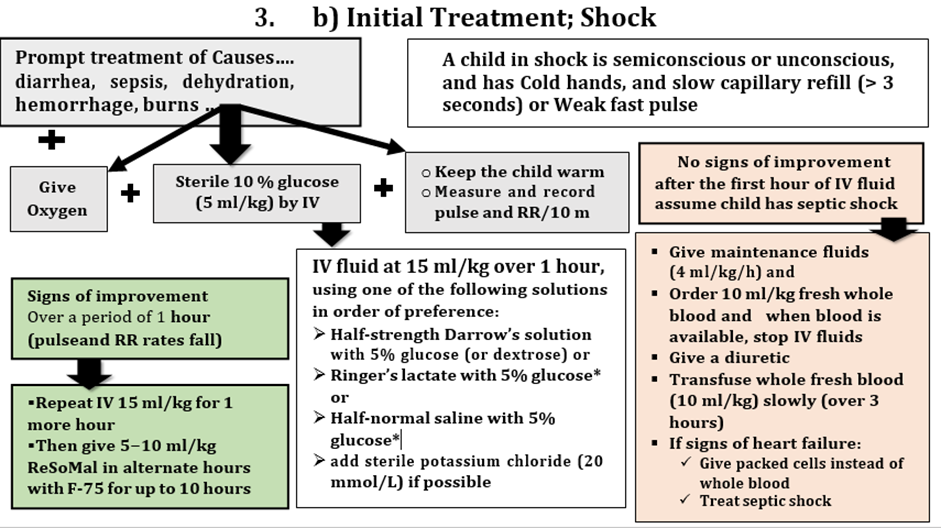
· Alertness: Conscious Semi-conscious Apathetic Unconscious Shock if Yes - Give Oxygen - Sterile 10 % glucose (5 ml/kg) by IV - Keep the child warm - measure and record pulse and RR/10 m - If improved over a period of 1 hour (pulse and RR rates fall) - Repeat IV 15 ml/kg for 1 more hour Then give 5−10 ml/kg ORS in alternate hours with F-75 for up to 10 hours · Attitude: Irritable Response to others Toxic look else |
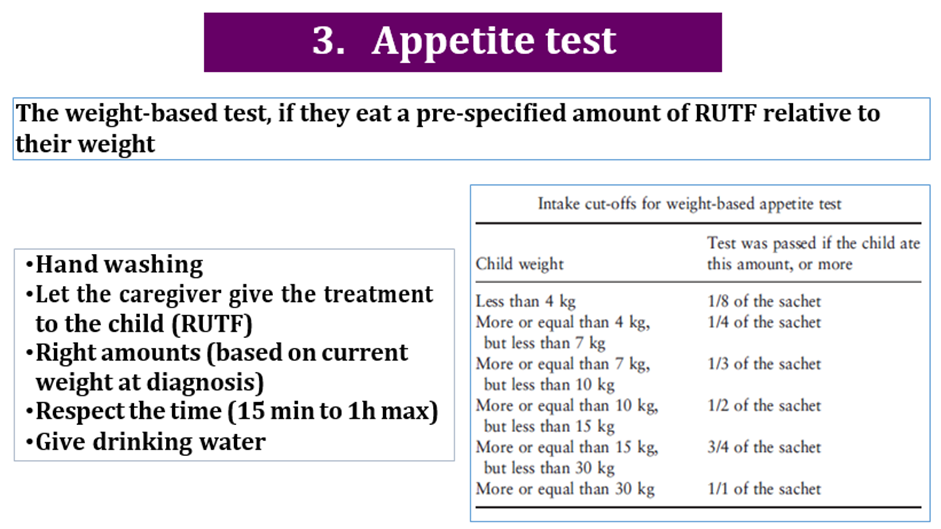
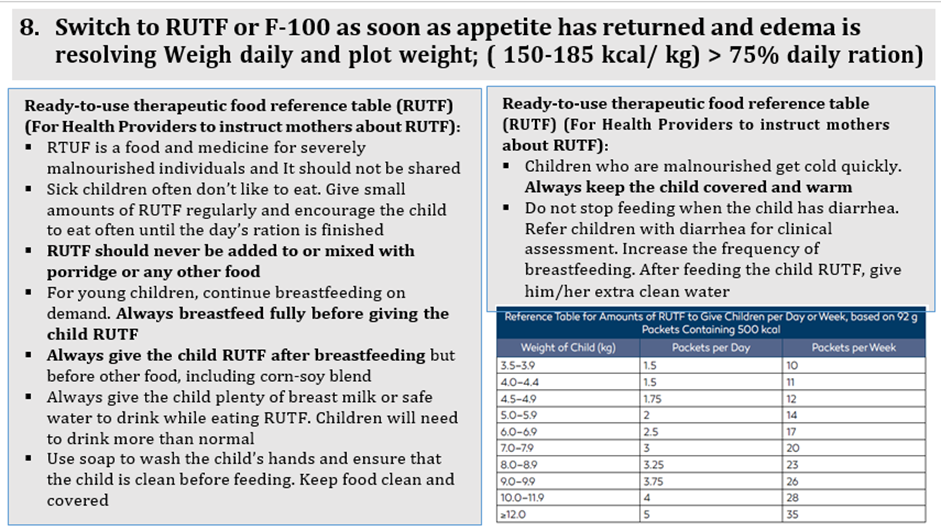
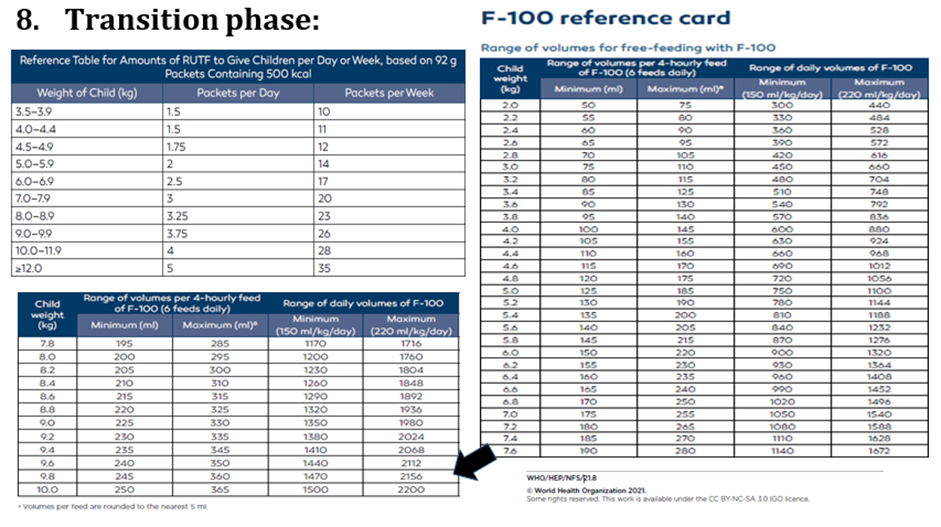
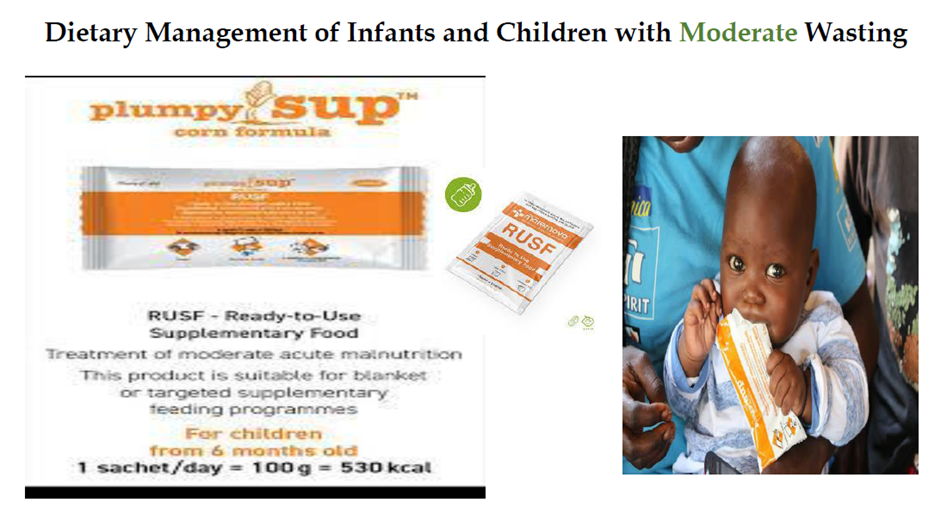
use RUT formulae according to schedule: amount that should be eaten = pass/fail amount left over
|
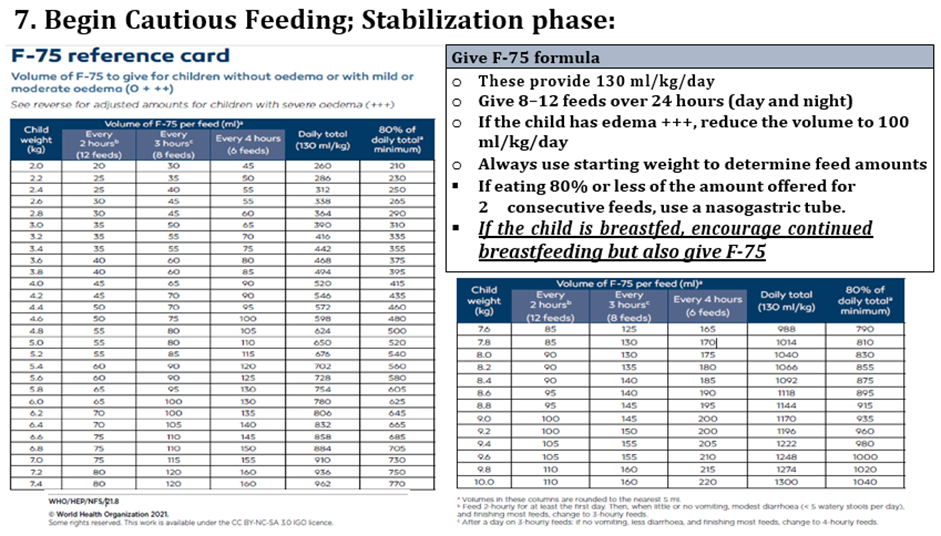
mg/dl (Hypoglycemia <54 mg/dl) - Give a bolus of 50ml (10% glucose) orally Or via nasogastric tube (if unable to drink), Or - (if unconscious) first give glucose (IV) (5 ml/kg of sterile 10% glucose) then nasogastric bolus - Start feeding with F-75 immediately following the Feeding schedule (2-hourly feeds) - For all children: Feed straight away and then every 2–3 hours, day and nigh
|
|
|
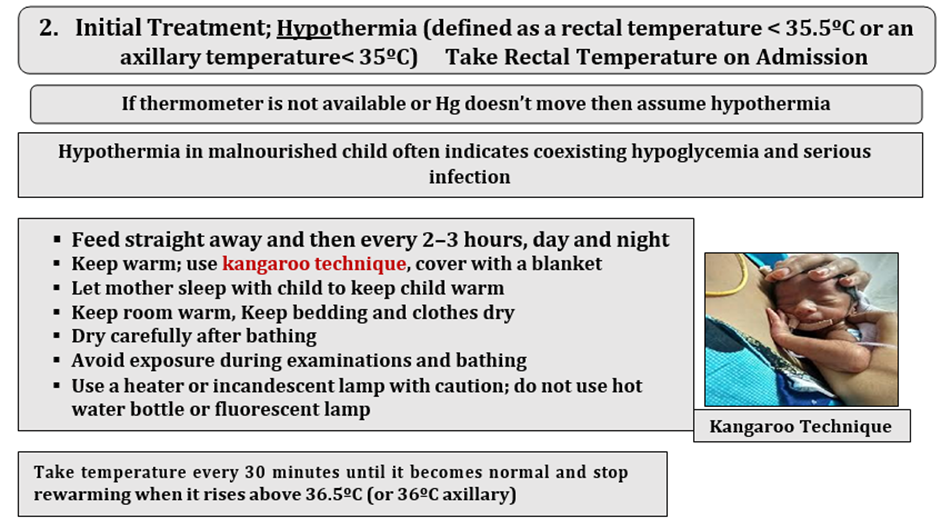
Hypothermia Rectal/axillary Take temperature every 30 minutes until it becomes normal and stop rewarming when it rises above 36.5ºC (or 36ºC axillary) Fever · Breathing: Distress Respiratory Rate: <2 months: < 60 breaths/minute** 2–11 months: < 50 breaths/minute** 12–59 months: < 40 breaths/minute |
Dietary History: · Weaned/not · Breast Feeds (complete Evaluation Sheet when appropriate)
· Correct positioning · ArtificialFormula No. /day Volume/Feed Scoops/30 ml · Supplementary Food Amount/d
|
Hb g/dl Anemia Septic Shock: Treat 1ry cause + § Give maintenance fluids (4 ml/kg/h) and § Order 10 ml/kg fresh whole blood and when blood is available, stop IV fluids § Give a diuretic § Transfuse whole fresh blood (10 ml/kg) slowly (over 3 hours) § If signs of heart failure: ü Give packed cells instead of whole blood ü Treat septic shock |
|
|
· Circulatory: Cold extremities Capillary refill Pulse Rate:
|
Frequency Pattern (For Food Groups) (When applicable) |
|
|
|
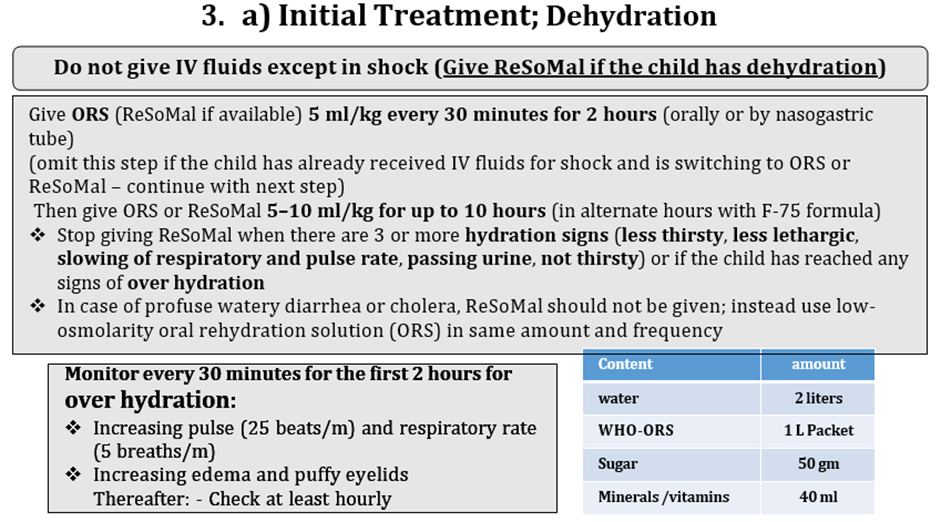
· Dehydration: Recent sunken Eye Skin integrity Tongue/mouth Tears Thirsty Eczema Skin infections/wounds Diarrhea watery bloody Vomiting Passed Urine If Dehydrated: § Give 5 ml/kg every 30 minutes for 2 hours (orally or by nasogastric tube) § (if the child has already received IV fluids for shock and is switching to ORS Then give ORS 5–10 ml/kg for up to 10 hours (in alternate hours with F-75 formula) § Stop giving ORS when there are 3 or more hydration signs (less thirsty, less lethargic, slowing of respiratory and pulse rate, passing urine, not thirsty) or § if the child has reached any signs of Over hydration: - Increasing pulse (25 beats/m) and respiratory rate (5 breaths/m) - Increasing edema and puffy eyelids Thereafter: - Check at least hourly
Physical Mental Delayed Growth Delayed Development |
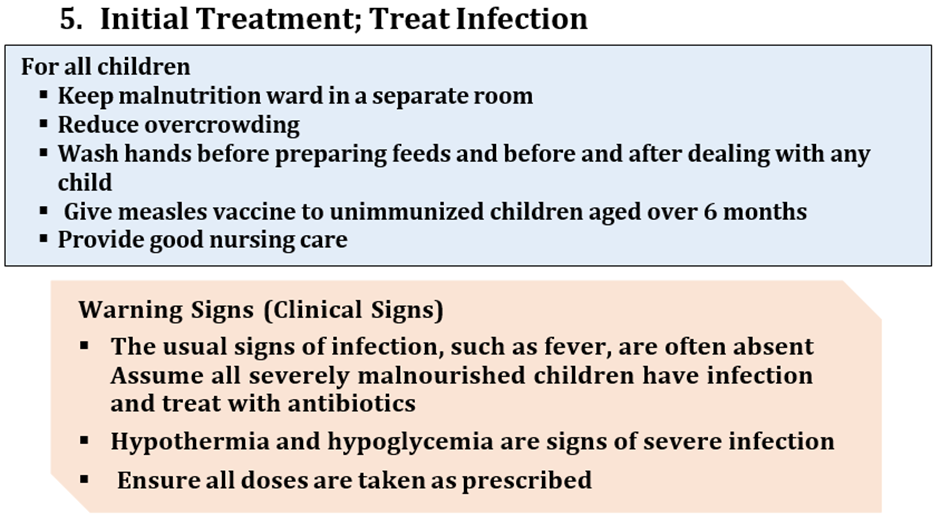
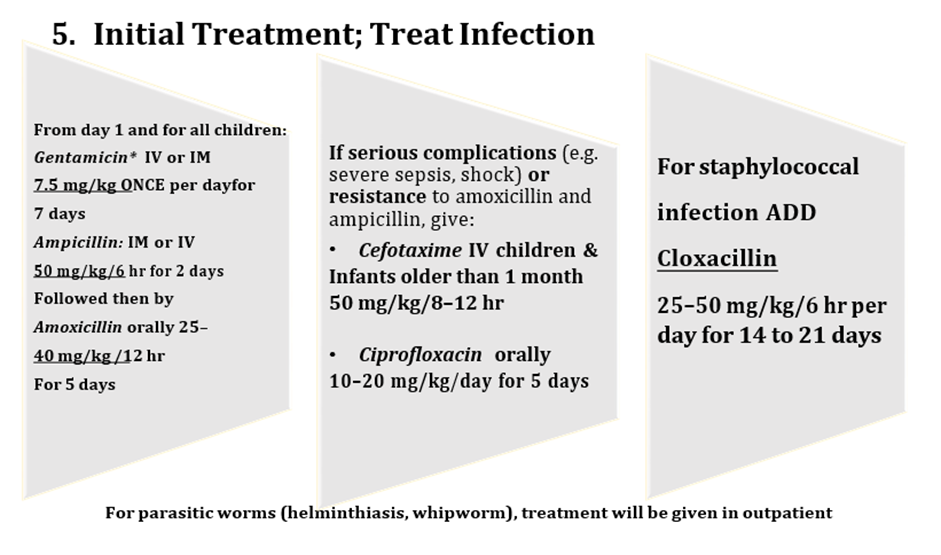
From day 1 and for all children: Gentamicin* IV or IM 7.5 mg/kg ONCE per day For 7 days Ampicillin:IM or IV 50 mg/kg/6 hr for 2 days Followed then by Amoxicillinorally 25–40 mg/kg /12 hr For 5 days If serious complications(e.g. severe sepsis, shock) orresistance to amoxicillin and ampicillin, give: • CefotaximeIV children & Infantsolder than 1 month 50 mg/kg/8–12 hr • Ciprofloxacinorally 10–20 mg/kg/day for 5 days For staphylococcal infection ADD Cloxacillin 25–50 mg/kg/6 hr per day for14 to 21 days
|
||
|
Plan for Feeding Support Breast Feeding as possible: - Mother/care giver - Relactation - Wet nursing Supplementary Food: No Edema:
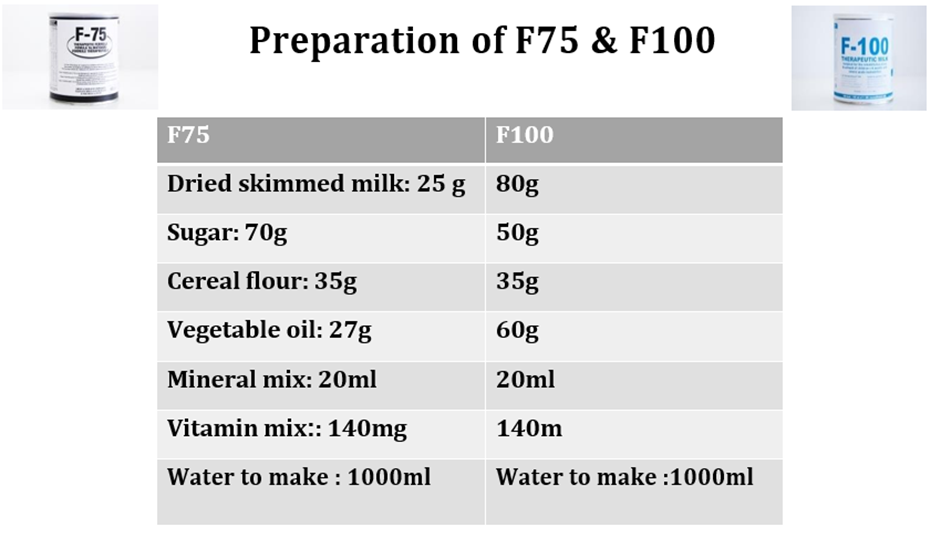
Edema: Commercial (generic) infant formula or F-75 (Full-strength F-100 should not be given if they are clinically unstable and/or have diarrhea or dehydration and/or nutritional edema (due to the renal solute load of this therapeutic milk and risk of hyponatremia dehydration) |
|||

Limitations and suggestions for further research needs
Future research recommendations for the management of Wasting in children in the Egyptian context could include:
· More epidemiological researches (determinants of acute malnutrition)
· Studies on alternative cheap, nutritious and local recipes
· Efficacy studies on the management protocols
These recommendations aim to address specific challenges and characteristics of the Egyptian context, potentially leading to more effective prevention and management strategies for Wasting in children.
Challenges
· Socially and economicallyunprivileged population
· Nutritional illiteracy
· Lack of enough trained healthcare workers to deal with these problems
· Limited resources
Strengthen the evidence base of the next update of this guideline by generating GRADE summary of finding tables, evidence profiles, and EtD frameworks.
Monitoring and evaluating the impact of the guideline.
The following are three performance measures or indicators for implementing this adapted CPG for Wasting in children:
1. Adherence to Wasting Guidelines
· Numerator: Number of children with wasting who received treatment as per guideline recommendations.
· Denominator: Total number of children diagnosed with wasting.
· Data Source: Hospital or clinic patient records.
2. Duration of Hospital Stay
· Numerator: Total number of hospital stay days for children with SAM
· Denominator: Total number of children admitted with SAM
· Data Source: Hospital admission and discharge records.
3. Rate of Readmission
· Numerator: Number of children readmitted with symptoms of SAM within a certain period (e.g., 30 days) after discharge.
· Denominator: Total number of children initially admitted with SAM.
· Data Source: Hospital readmission records.
These key performance indicators are designed to measure the effectiveness and adherence to the guidelines, the efficiency of the treatment in terms of resource utilization (hospital stay), and the success of the treatment in preventing further complications (readmissions).
Updating of the guideline
The EPG WGAG has decided to conduct the next review of this adapted CPG for updates after three years. This should be carried out in 2027 after checking for updates in the source CPGs, consultation of expert opinion on the changes needed for updating according to the newest evidence and recommendations published in this area and the clinical audit and feedback from implementation efforts in the forementioned local healthcare settings except if any breakthrough evidence- based recommendations are published before that date. The updating will be guided by the Checklist for the Reporting of Updated Guidelines (CheckUp) that is one of the AGREE Tools
