
Cochlear Implantation: Phoniatric perspective
"last update: 27 August 2024"
- Annexes
Editorial Independence:
▪️ This guideline was developed without any external funding.
▪️ All the guideline development group members have declared that they do not have any competing interests.
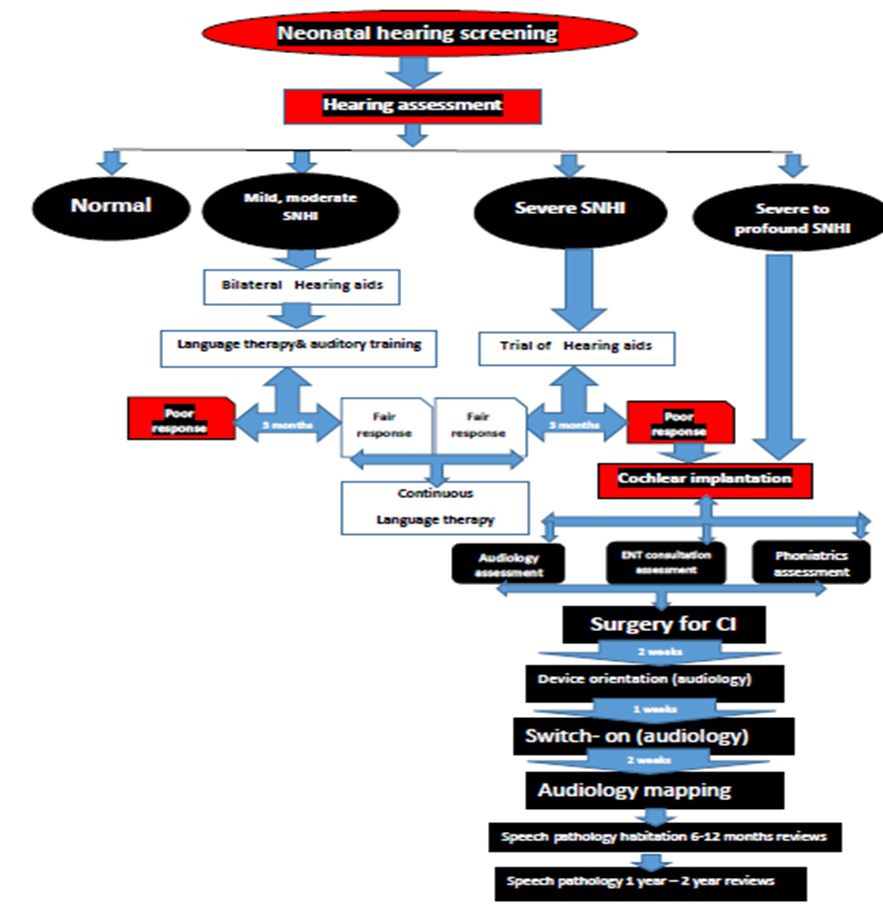
Annex1: Guideline Flowchart
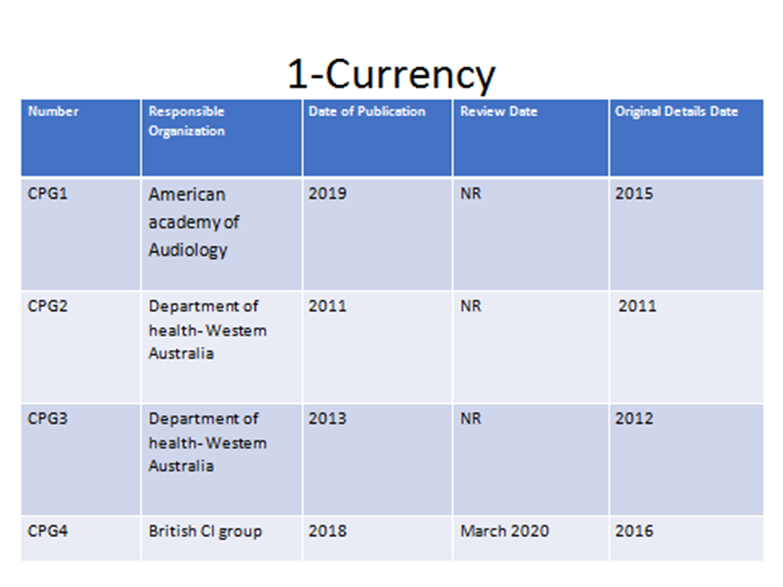
Annex2: Tables of appraisal of selected guidelines:Currency (table 1), Content (table 2) and Quality (table 3) of the selected guidelines.

|
Criteria |
Guideline 1 |
Guideline 2 |
Guideline 3 |
Guideline 4 |
|
Clinical practice guidelines 2019 |
Neuroscience 2011 (Pediatrics) |
Neuroscience 2013 (Adults) |
British CI 2018 |
|
|
Credibility |
9 |
6 |
6 |
8 |
|
Observability |
6 |
3 |
3 |
5 |
|
Relevance |
7 |
8 |
8 |
8 |
|
Relative advantage |
7 |
7 |
7 |
7 |
|
Easy to install and understand |
8 |
7 |
7 |
8 |
|
Compatibility |
8 |
6 |
6 |
8 |
|
Testability |
8 |
8 |
8 |
8 |
|
Total |
53 |
45 |
45 |
52 |

|
Domain |
CPG1 |
CPG2 |
CPG3 |
CPG4 |
|
Transparency |
A |
A |
A |
A |
|
Conflict of Interest |
NR |
NR |
NR |
NR |
|
Development Group |
A |
C |
C |
B |
|
Systematic Review |
A |
B |
B |
A |
|
Grade of Evidence |
A |
B |
B |
B |
|
Recommendations |
A |
B |
B |
B |
|
External Review |
C |
C |
C |
A |
|
Update |
B |
B |
B |
A |
|
CPG1: 5A, 1B, 1C, 1NR |
||||
Annex3: The risks and benefits of added and/or modified statements
|
Statement |
Risk |
Benefit |
|
The progress of children with other comorbidities should be measured by criteria that are unique to them and that reflect the goals of the family. |
Low family goals expected from implanting those children, can affect the outcomes. |
Those children should not be excluded from candidacy. They can benefit from implantation, with counselling given towards realistic expectations. |
|
Bilateral stimulation should be considered for all individuals who use a cochlear implant, if not otherwise contraindicated. |
No risk |
Bilateral stimulation should be considered for all individuals who use a cochlear implant, all of its benefits. |
