The Embryos to Transfer in IVF/ICSI
"last update: 6 August 2024"
- Methods
A comprehensive search for guidelines was undertaken to identify the most relevant guidelines to consider for adaptation.
Inclusion/exclusion criteria followed in the search and retrieval of guidelines to be adapted:
● Selecting only evidence-based guidelines (guideline must include a report on systematic literature searches and explicit links between individual recommendations and their supporting evidence)
● Selecting only national and/or international guidelines
● Specific range of dates for publication (using Guidelines published or updated 2010 and later)
● Selecting peer reviewed publications only
● Selecting guidelines written in English language
● Excluding guidelines written by a single author not on behalf of an organization in order to be valid and comprehensive, a guideline ideally requires multidisciplinary input
● Excluding guidelines published without references as the panel needs to know whether a thorough literature review was conducted and whether current evidence was used in the preparation of the recommendations
The following characteristics of the retrieved guidelines were summarized in a table:
• Developing organization/authors
• Date of publication, posting, and release
• Country/language of publication
• Date of posting and/or release
• Dates of the search used by the source guideline developers
All retrieved Guidelines were screened and appraised using AGREE II instrument (www.agreetrust.org) by at least two members. the panel decided a cut-off point or rank the guidelines (any guideline scoring above 50% on the rigor dimension was retained)
These Guidelines were adapted mainly from” performing the embryo transfer: a guide” Practice committee of the American Society of Reproductive medicine 2017,“1ESHRE guidelines: number of embryos to transfer during IVF/ICSI” developed by the ESHRE guideline group on the number of embryos to transfer in 20242 and “Guidance on the limits to the number of embryos to transfer” developed by the Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society of Assisted Reproductive Technology in 20213.
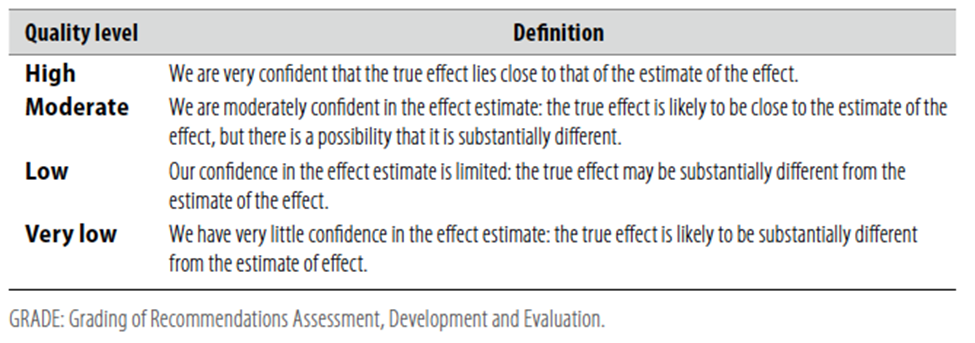
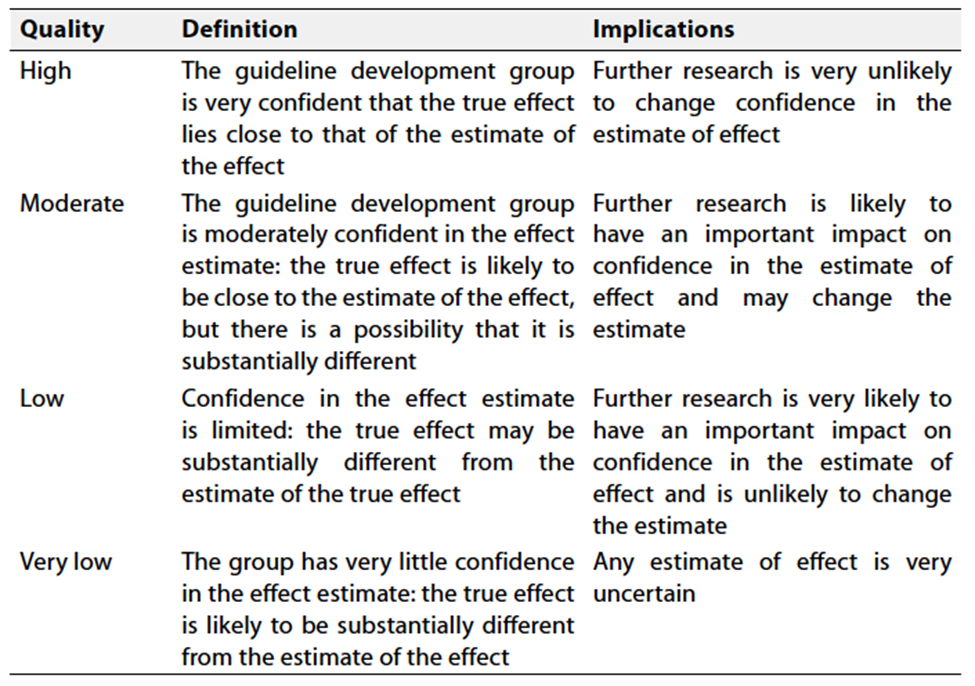
According to WHO handbook for Guidelines we used the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach to assess the quality of a body of evidence, develop and report recommendations. GRADE methods are used by WHO because these represent internationally agreed standards for making transparent recommendations. Detailed information on GRADE is available on the following sites:
■ GRADE working group: http://www.gradeworkingroup.org
■ GRADE online training modules: http://cebgrade.mcmaster.ca/
■ GRADE profile software: http://ims.cochrane.org/revman/gradepro
Table 1 Quality of evidence in GRADE