
Equine sampling
- Cerebrospinal fluid sampling
Aim
- Analysis of cerebrospinal fluid may provide clues that when combined with history, physical examination, and other diagnostic procedures, may aid in diagnosis or management of horses with neurologic disease.
- Cerebrospinal fluid should be collected from the site closest to the lesion, if possible. the site of sampling are Atlanta-occipital cistern or lumbo-sacral cistern.
- The site of lumbo-sacral cistern is the site preferred to collect CSF in horses with signs of spinal cord disease.
- the atlantooccipital cistern is the site preferred to collect CSF from horses with signs of brain disease.
- Because of the lack of sensitivity in CSF analysis, collecting CSF from the appropriate site may improve the chances that analysis will provide useful information.
Contraindications
1- CSF should not be collected from horses suspected of having rabies.
2- Collection of CSF is contraindicated for horses with head trauma or metabolic disease that have signs of significant cerebral edema (such as dilated pupils and an altered state of consciousness). Removal of CSF from a horse with elevated intracranial pressure may cause a rapid decrease in CSF pressure resulting in death from herniation of brain tissue through the foramen magnum.
3- Collection of CSF from the atlanto-occipital cistern requires general anesthesia and therefore contraindicated for horses that are at risk of dying under general anesthesia, or of being unable to regain a standing position.
4- Puncture of the dura mater by the spinal needle is painful and may provoke an extreme reaction in the standing horse, which could cause injury to the clinician or horse.
5- Collection of CSF from the lumbo-sacral cistern is contraindicated if the temperament of the horse or an inadequate facility to restrain the horse jeopardizes the safety of the clinician or horse.
6- The optimal site for sampling is determined on the basis of the neuroanatomical localization of the suspected lesion and practical.
Technique of CSF sampling at Lumbosacral site
1. Site preparation A 10 x 10 cm site is clipped and sterilely prepped.
2. Positioning Landmarks for the LS site are the intersection of the midline with either a line joining the caudal borders of the tuber coxae, or the highest point of the gluteal region of the horse.
3. local anesthetic solution is used to collect fluid from the lumbo-sacral cistern.
4. Sedation may dampen the reaction to puncture of the dura mater at the lumbo-sacral site in the standing horse, but sedation may compound the difficulty of persuading an already ataxic horse to stand squarely to facilitate accurate placement of the needle. To dampen the reaction to puncture of the dura mater, a lip twitch should be applied to a standing horse, even if the horse is sedated.
5. Alignment of the pelvis may be aided by elevating the upper pelvic limb to make the tuber coxae perpendicular to the ground, and advancing the pelvic limbs to flex the pelvis opening the LS space.
6. Puncturing the skin with a #11 blade or a large bore needle reduces drag on the spinal needle as it is advanced.
7. A sterile 20-gauge 6-inch (15.2 cm) spinal needle with stylet is inserted in a sterile manner and advanced carefully a few millimeters at a time. Care should be taken to keep the needle perpendicular to the dorsum and on midline. A “popping” sensation may be felt with penetration of the LS interarcuate ligament, dorsal dura mater, and arachnoid membrane. A needle depth of 12 to 14 cm is commonly required for successful CSF collection. Responses of sedated horses to penetration of the dura mater range from no reaction to tail movement and slight flexion of the pelvic limbs to violent kicking that can endanger the patient and the veterinarian.
8. The stylet is removed to check for CSF at the hub. Gentle aspiration with a syringe may be necessary to initiate flow of spinal fluid. If no fluid is obtained, the needle (with the stylet replaced) is advanced to the floor of the vertebral canal and then withdrawn with slow rotation of the needle a millimeter at a time.
9. After adequate CSF is obtained, the stylet is replaced in the spinal needle and the needle is removed.
10. Microproteins can now be accurately measured in CSF collected into EDTA tubes. Because the low protein concentration of CSF causes cellular destruction, CSF collected in plain glass tubes should be examined within 30 minutes. Enrichment media for bacterial culture, if indicated.Cold packs for shipment of samples. CSF should be kept cold for shipment. Cytology of CSF collected in EDTA tubes should be performed within 24 hours.
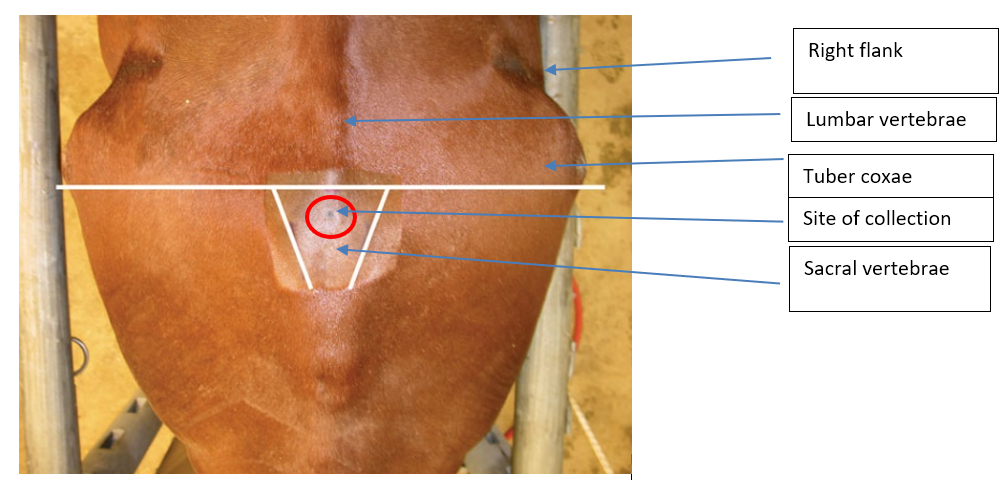
The site for collection of CSF at the lumbo-sacral cistern is identified on the midline between and near the cranial borders of the tuber sacral or near a line drawn between the caudal borders of the tuber coxae on the midline. These landmarks are not easily found on a well-conditioned horse.
