Equine sampling
| Site: | EHC | Egyptian Health Council |
| Course: | Equine Medicine Guidelines |
| Book: | Equine sampling |
| Printed by: | Guest user |
| Date: | Monday, 23 December 2024, 10:01 PM |
Table of contents
- - Committee
- - Scope
- - Introduction
- - Blood sample
- - Blood diagnostic tests
- - Nasopharyngeal swab collection
- - Tracheal Washes
- - Uterine swab in mare
- - Fecal sample
- - Skin scrapings
- - Peritoneal fluid collection (Abdominocentesis)
- - Synovial Fluid sampling
- - Cerebrospinal fluid sampling
- - Samples from fetus
- - References
- Committee
We would like to acknowledge the committee of National Egyptian Guidelines for Veterinary Medical Interventions, Egyptian Health Council for adapting this guideline.
Executive Chief of the Egyptian Health Council: Prof. Dr Mohamed Mustafa Lotief.
Head of the Committee: Prof. Dr Ahmed M Byomi
The Rapporteur of the Committee: Prof. Dr Mohamed Mohamedy Ghanem.
Scientific Group Members: Prof. Dr Nabil Abd Elgaber, Prof. Dr Ashraf Aldesoky Shamaa, Prof. Dr Amany Abbas, Prof. Dr Dalia Mansour, Dr Essam Sobhy, Dr Mohamed Elsharkawi.
Editor: Dr Mohamed Elsharkawi
- Scope
This guideline outlines the most common methods with its precaution appropriate for collecting samples for diagnoses of the most equine diseases including infectious, non-infectious and zoonotic diseases. For this manual to become an effective document, it is important that the proposed strategies are incorporated into routine protocols and staff training.
The Target audience
The guideline is intended for all veterinarians dealing with equine population and Who are working at veterinary clinics and equine farms owners.
- Introduction
Diagnostic tests are essential tools for confirming the health status of animals and identifying pathogens. They enable the early detection, management and control of animal diseases including zoonosis, it is important to take into account several basic aspects of the sampling for the laboratory analysis in order to avoid mistakes in this stage which could lead to false conclusions drawn from the interpretation of results:
1. Choosing the correct and relevant material when carrying out the sampling.
2. Two samples collected in clean/sterile conditions depending on the case.
3. The samples taken should be transported to the laboratory in ice box for the detection of the antigen.
4. The serum samples must be collected for the detection of antibodies (serology).
5. it is very important to label the samples correctly.
A detailed case report should be included with the samples
1. Owner's name – farm name
2. Horse ID.
3. Owner’s complains and clinical signs
4. Gross findings and lesions (including size and anatomic location).
5. previous treatment, including response to treatment (if any) results of relevant diagnostic testing, such as CBC, serum biochemical analysis, cytologic and histologic evaluation, and imaging studies
6. Previous vaccination program
7. Feeding program
The most common equine samples are:
1- Blood sample.
2- Nasopharyngeal swab.
3- Tracheal Washes.
4- Uterine swab in mares.
5- Fecal sample.
6- Skin scrapings.
7- Peritoneal fluid (Abdominocentesis).
8- Synovial Fluid (Arthrocentesis).
9- Cerebrospinal fluid sampling technique.
10- Samples from fetus.
- Blood sample
Blood work can recognize an abnormality before you even see clinical signs of specific disease, thus protecting your horse and other horses.
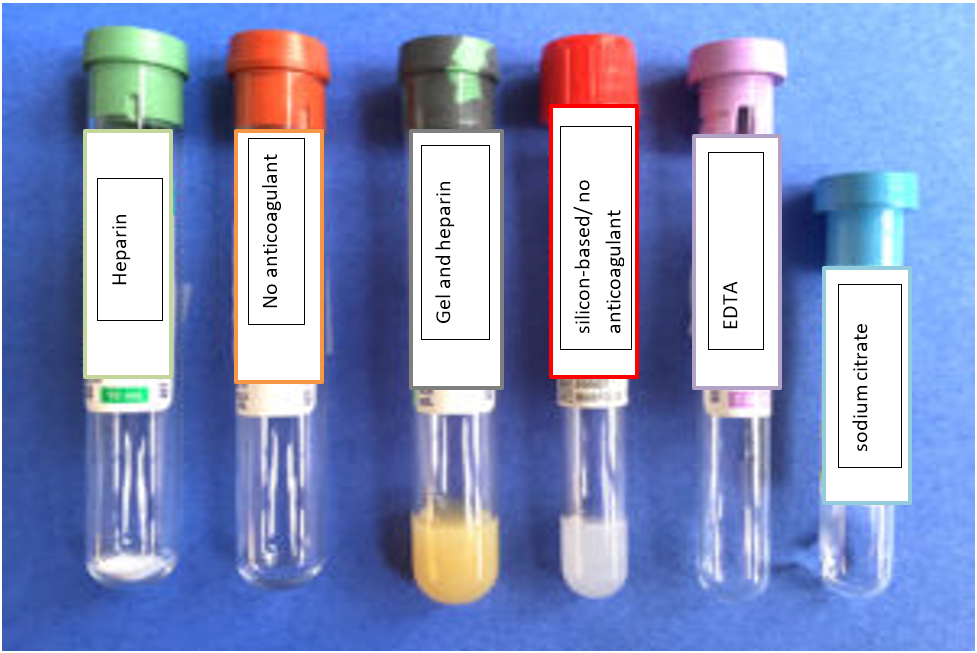
Types of Blood Collection Tubes
1) Green top tube containing heparin (yields plasma for biochemistry).
2) Red top tube with no anticoagulant (yields serum for biochemistry).
3) Tiger strip green top tube containing gel and heparin or plasma separator tube (yields plasma and makes separation of red blood cells easier with centrifugation, but hardly ever used, always separate plasma from the silicon tube and put in a plain or non-anticoagulant tube).
4) Silicone-based or serum separator red top tube with no anticoagulant (used frequently for large animals, which take a while to undergo clot retraction, silicon makes it easy to separate serum and cells, however serum should be removed off the silicone gel and placed in a plain or non-anticoagulant tube.
5) Purple top tube containing EDTA as an anticoagulant (used for hematology and cytology on body cavity fluids).
6) Blue top tube containing sodium citrate as an anticoagulant (for obtaining plasma for coagulation panel testing, collect appropriate volume of blood for the amount of citrate, separate plasma from cells and ship on ice or freeze plasma and ship on dry ice).
- Blood diagnostic tests
1- Hematology
This type of test examines the cells in the blood which are divided into red blood cells, white blood cells and platelets. Information on how many cells, their size and any apparent abnormalities in the cells. Additionally, the different subtypes of white blood cells are counted including neutrophils, lymphocytes, monocytes eosinophils and basophils.
2- Biochemistry
There are many chemical substances that circulate in the blood stream that can be used to indicate damage or disease in various organs as follow:
a) When we see higher concentrations of certain biochemical markers in the blood, it may indicate that the liver cells for example have been damaged and have released their contents into the blood stream (such as ALT, AST and ALP).
b) Some enzymes such as CK should be located within muscle cells and therefore a high level in the blood stream indicates damage to muscle cells.
c) Chemicals if found in the blood can imply that certain organs are not functioning correctly. For example, the kidneys should remove creatinine and urea from the blood stream and therefore higher levels suggest that kidney function is impaired.
d) The higher levels of bilirubin and bile acids indicate that the liver is not fully functional.
3- Serology
- Serology is the measurement of antibody levels in the blood stream. Serology testing can be confusing as it is one type of test where a positive result doesn’t necessarily mean that the horse has the disease, and a negative result does not rule out the disease!
- It is perfectly possible to have a positive result (a high antibody level) due to exposure to a disease several years previously from which the horse has now recovered. Additionally, it takes a couple of weeks or so to develop an antibody response and so a horse that has been exposed to an infectious disease within the last week may well still test negative.
4- Endocrinology
This refers to measurement of hormone levels in the blood, for example Equine Metabolic Syndrome is a further condition making horses and ponies prone to laminitis. often in association with being overweight and inactive. This is usually diagnosed and investigated via measurement of the hormones for example insulin and adiponectin which both indicate increased laminitis risk.
5- Blood parasites
Infected horses can be identified by demonstrating the parasites in stained blood or organ smears during the acute phase of the disease. Romanovsky-type staining methods, such as Giemsa, give the best results. In carrier animals, low parasitaemias make it extremely difficult to detect parasites, especially in the case of B. caballi infections, although they
may sometimes be demonstrated by using a thick blood smear technique.
Procedure of blood collection:
1. Personal Protective Equipment (PPE) and Hygiene. Ensure appropriate PPE is used to protect handler from accidental injury or exposure to blood and other body fluids.
2. Restrain animal
3. Clip (optional) and swipe with antiseptic gauze to remove superficial dirt and debris. This may also assist in visualizing raised vein.
4. Occlude jugular vein by applying pressure in the jugular groove 2-3 inches below venipuncture site and visualize raised vein.
5. With bevel up, insert needle firmly and smoothly (don’t jab) through the skin and into the vein at 20°angle.
6. When using needle and syringe, break the seal on the syringe by gently pulling back before use.
7. If using vacutainer, once needle inserted, stabilize needle and push the vacutainer tube into hub. If you have hit the vein, blood will flow freely into tube. Multiple tubes can be filled by removing filled tube and replacing with fresh tube.
8. Serial samples can be taken by alternating sides, and by moving insertion sites cranially, as long as there is no hematoma formation.
Potential Adverse Effects of blood collection
a. Hematoma or thrombus
b. Pain at blood collection site
c. Infection at blood collection site Ch. by:
1. Heat, pain, swelling first noted at the insertion site of the blood draw, purulent material draining from the insertion site.
2. Induration (hardening) of the vessel.
3. Pyrexia, local or systemic infections, septic shock.
- Nasopharyngeal swab collection
Aim of collecting nasopharyngeal swab
To make confirmative diagnosis of specific infection of nasopharynex.
Material:
• Dacron Swabs with plastic sticks 5—7 inches (often supplied with bacterial transport media)
• Plain red top tube (blood tube, no additive) or viral transport media.

Nasopharyngeal swab (external matching site).
Procedure
1. The horse should be restrained sufficiently to introduce swab in a manner safe for horse and attending personnel.
2. If sampling more than one horse, fresh gloves should be used for each horse.
3. Pass the swab(s) along the left or right ventral meatus being careful to avoid the false nostril (nasal diverticulum), until you are in the horse’s nasal passage. The distance is approximately similar to that from the nostril to the eye. Swallowing indicates the correct depth of swab penetration Rotate the swab to increase the collection of respiratory secretions for at least 5 seconds. Two swabs can be done at the same time if more than one sample is needed by the laboratory. Some labs request one sample for qPCR and one for viral isolation. The dirt often inhibits PCR analysis. Alternatively, clean the nostril to be collected with a disposable cloth prior to swab collection.
5. Consider all waste materials and protective equipment to be infectious (gloves, gowns, packaging and swabs).
6. Any restraining equipment such as twitches, nose chains, and lead ropes must be disinfected between uses.
7. Keep tubes refrigerated and ship on ice overnight to a diagnostic laboratory of your choice.
- Tracheal Washes
Aim:
- To determine the number of nucleated cells in the sample as macrophages and neutrophils, which are present at different stages of a bacterial infection, A high nucleated cell count is often suggestive of an active infection.
- Also culture tracheal washes on agar plates, then incubated for 24 hours. This encourages any bacteria present in the tracheal wash fluid to grow and identify them. , antibiotic sensitivity will be carried out to prescribe the most effective antibiotic to get rid of the infection.
Technique
- Tracheal wash can perform by using an endoscope – a long tube through which a small amount of liquid (ex. Sterile saline, dist. Water) is flushed into the trachea and then sucked back into a syringe, which is then submitted to the lab.
- Uterine swab in mare
Aim: uterine swabs must be collected for detection of:
- Venereal disease control.
- Identification of infected/carrier mares.
- Optimize reproductive efficiency.
- Satisfy industry pre-breeding requirements.
Technique
A guarded, sterile swab is used to obtain samples for uterine culture. With the mare in stocks, the tail bandage and the perineum washed, the culture rod is introduced into the vagina with a gloved hand. After the rod is guided through the cervix, the guard cap is dislodged and the swab is rubbed along the endometrium, after which the rod is extracted. Samples for uterine culture should only be obtained during full estrus. Swabs should be directly plated onto agar within 2 hours of collection.
- Fecal sample
Aim:
Performing regular fecal egg count testing is an important step in equine parasite management.
Procedure:
1. Use a clean new plastic glove for each sample. Protect gloves and containers from inadvertent fecal or dirt contamination when sampling in barn. Collect the fecal sample directly from the rectum with care to avoid rectal injury or perforation which leading to peritonitis.
2. Store in a cool place, such as a fridge.
3. Deliver your fecal sample to the vet within 48 hours!
Note:
Do not place the sample in the freezer or leave it in your car. Extreme cold or heat can kill parasites.
- Skin scrapings
Aim:
Skin scraping is a diagnostic procedure that involves scraping of skin with a scalpel blade or curette to detect the presence of different skin lesions and microscopic ectoparasites, to detect mites, larvae of Pelodera spp., and Habronema spp. equine skin conditions very superficial and comprise scale that will not commonly enable to diagnosis.
Procedure
1. Gently clip the hair in area for sampling Select the area to be scraped.
2. Place a drop of mineral oil on the blade. Another drop can also be placed on the area of skin to be sampled.
3. Scrape with the scalpel blade (keeping the blade perpendicular to the skin) in the direction of hair growth several times until a small amount of skin surface debris is obtained.
4. Deeper scrapes can be performed by pinching the skin between finger and thumb and using a scalpel blade to scrape the skin until capillary bleeding occurs.
5. Superficial skin scrapings may be helpful in the diagnosis of dermatophytosis. Scrapings are best taken from the periphery of the lesions where the arthrospores are more likely to be visible microscopically.
6. Placing the sample in 10% potassium hydroxide will destroy a lot of the debris, making it easier to identify fungal components. Scrapings from the distal limbs are useful in the identification of Chorioptes mites . Removal of superficial crusts for microscopy and bacterial culture enables diagnosis of dermatophilosis (rain scald).
- Peritoneal fluid collection (Abdominocentesis)
Aim:
- Peritoneal fluid analysis to confirm diagnosis of intra-abdominal pathology including inflammatory, infectious, neoplastic, obstructive, and bowel strangulation, leading to additional diagnostic and therapeutic plans as in horses suffering from colic, diarrhea, weight loss, or other conditions involving the abdominal cavity and is an integral component of diagnostic testing for colic.
- To evaluate the abdominal cavity of the horse with valuable diagnostic information to monitor patients with abdominal diseases being managed medically and to determine their need for surgical management.
Procedure
1. Abdominal fluid collection using an 18-Ga, 3.8-cm (1.5-in) needle is recommended for adult horses to penetrate the peritoneal cavity. The teat cannula technique is recommended for use in adult horses, foals, and miniature horses to reduce the risk of enterocentesis, even though this procedure is more traumatic than using an 18-Ga, 3.8-cm needle.
2. The needle or teat cannula is manipulated for several minutes in an attempt to obtain a sample from an area of fluid accumulation.
3. The sample is collected into an ethylene diaminetetraacetic acid (EDTA) tube for cytology and a sterile tube, syringe, or culture vial for bacterial culture and sensitivity testing.
Complications
1- The main complication with abdominocentesis is enterocentesis, which should be suspected when green-brown fluid is obtained in a horse with clinical signs inconsistent with gastrointestinal tract rupture/perforation the incidence rate about 2–5% was reported . When an enterocentesis occurs, the needle or teat cannula should be removed. Although prophylactic antimicrobial drug administration is recommended, the necessity is unknown.
2- Localized cellulitis and peritonitis are rare consequences of enterocentesis and probably more likely to occur in horses with compromised bowel.
3- Injury to the spleen can occur and may happen more frequently in horses with a nephrosplenic entrapment whereby the spleen is moved ventrally and across midline. .
4- Omental herniation is a complication of abdominocentesis in foals using the teat cannula Abdominocentesis in foals should be performed with sonographic guidance and using a needle
- Synovial Fluid sampling
Aim:
The evaluation of synovial fluid in equine medicine was used for
1. The diagnosis of equine lameness and joint disease.
2. To evaluation the systemic diseases in which joint effusion is part of the clinical picture.
Procedure
1. The skin should be prepared by clipping the hair and performing a surgical scrub to avoid introducing bacteria into the joint.
2. Ethyl chloride spray may be used, if needed, on the overlying skin to lessen the sensation of the needle penetration.
3. Synovial fluid can be aspirated from most joints with a 1-inch, 18- to 20-gauge needle.
4. Fluid for cytologic evaluation should be placed in an EDTA tube. However, that EDTA interferes with the mucin clot test. If a mucin clot test is to be run, another aliquot of synovial fluid should be placed in a tube with no anticoagulant (red-top tube) or one containing heparin.
5. The three most common problems associated with intra-articular injection in horses are septic arthritis, flare reactions and periarticular cellulitis.
- Cerebrospinal fluid sampling
Aim
- Analysis of cerebrospinal fluid may provide clues that when combined with history, physical examination, and other diagnostic procedures, may aid in diagnosis or management of horses with neurologic disease.
- Cerebrospinal fluid should be collected from the site closest to the lesion, if possible. the site of sampling are Atlanta-occipital cistern or lumbo-sacral cistern.
- The site of lumbo-sacral cistern is the site preferred to collect CSF in horses with signs of spinal cord disease.
- the atlantooccipital cistern is the site preferred to collect CSF from horses with signs of brain disease.
- Because of the lack of sensitivity in CSF analysis, collecting CSF from the appropriate site may improve the chances that analysis will provide useful information.
Contraindications
1- CSF should not be collected from horses suspected of having rabies.
2- Collection of CSF is contraindicated for horses with head trauma or metabolic disease that have signs of significant cerebral edema (such as dilated pupils and an altered state of consciousness). Removal of CSF from a horse with elevated intracranial pressure may cause a rapid decrease in CSF pressure resulting in death from herniation of brain tissue through the foramen magnum.
3- Collection of CSF from the atlanto-occipital cistern requires general anesthesia and therefore contraindicated for horses that are at risk of dying under general anesthesia, or of being unable to regain a standing position.
4- Puncture of the dura mater by the spinal needle is painful and may provoke an extreme reaction in the standing horse, which could cause injury to the clinician or horse.
5- Collection of CSF from the lumbo-sacral cistern is contraindicated if the temperament of the horse or an inadequate facility to restrain the horse jeopardizes the safety of the clinician or horse.
6- The optimal site for sampling is determined on the basis of the neuroanatomical localization of the suspected lesion and practical.
Technique of CSF sampling at Lumbosacral site
1. Site preparation A 10 x 10 cm site is clipped and sterilely prepped.
2. Positioning Landmarks for the LS site are the intersection of the midline with either a line joining the caudal borders of the tuber coxae, or the highest point of the gluteal region of the horse.
3. local anesthetic solution is used to collect fluid from the lumbo-sacral cistern.
4. Sedation may dampen the reaction to puncture of the dura mater at the lumbo-sacral site in the standing horse, but sedation may compound the difficulty of persuading an already ataxic horse to stand squarely to facilitate accurate placement of the needle. To dampen the reaction to puncture of the dura mater, a lip twitch should be applied to a standing horse, even if the horse is sedated.
5. Alignment of the pelvis may be aided by elevating the upper pelvic limb to make the tuber coxae perpendicular to the ground, and advancing the pelvic limbs to flex the pelvis opening the LS space.
6. Puncturing the skin with a #11 blade or a large bore needle reduces drag on the spinal needle as it is advanced.
7. A sterile 20-gauge 6-inch (15.2 cm) spinal needle with stylet is inserted in a sterile manner and advanced carefully a few millimeters at a time. Care should be taken to keep the needle perpendicular to the dorsum and on midline. A “popping” sensation may be felt with penetration of the LS interarcuate ligament, dorsal dura mater, and arachnoid membrane. A needle depth of 12 to 14 cm is commonly required for successful CSF collection. Responses of sedated horses to penetration of the dura mater range from no reaction to tail movement and slight flexion of the pelvic limbs to violent kicking that can endanger the patient and the veterinarian.
8. The stylet is removed to check for CSF at the hub. Gentle aspiration with a syringe may be necessary to initiate flow of spinal fluid. If no fluid is obtained, the needle (with the stylet replaced) is advanced to the floor of the vertebral canal and then withdrawn with slow rotation of the needle a millimeter at a time.
9. After adequate CSF is obtained, the stylet is replaced in the spinal needle and the needle is removed.
10. Microproteins can now be accurately measured in CSF collected into EDTA tubes. Because the low protein concentration of CSF causes cellular destruction, CSF collected in plain glass tubes should be examined within 30 minutes. Enrichment media for bacterial culture, if indicated.Cold packs for shipment of samples. CSF should be kept cold for shipment. Cytology of CSF collected in EDTA tubes should be performed within 24 hours.
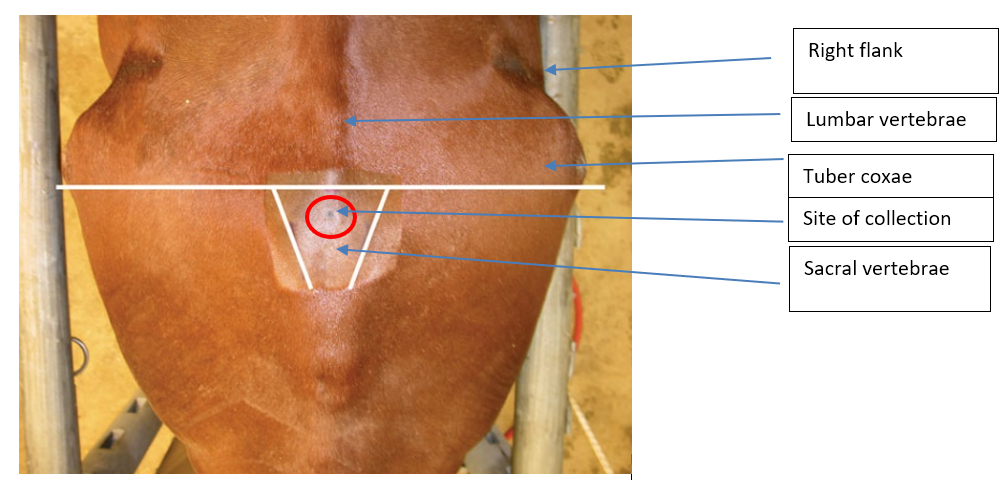
The site for collection of CSF at the lumbo-sacral cistern is identified on the midline between and near the cranial borders of the tuber sacral or near a line drawn between the caudal borders of the tuber coxae on the midline. These landmarks are not easily found on a well-conditioned horse.
- Samples from fetus
Necropsy
Straw and dirt were initially rinsed from the fetus using low pressure tap water. Weighed, measured (crown-rump length), and fetal developmental characteristics and gender were recorded. The fetus was inspected for external lesions and then opened in the ventral midline. Specimens were taken for polymerase chain reaction (PCR) analysis and microbial culture before further handling of the fetus using sterile equipment and new examination gloves and placed in separate plastic containers. The necropsy was then completed and tissues sampled for histopathology.
Specimens for histopathology were sampled from the allantochorion of each horn (midpart) and the body near the cervical star. If gross lesions were observed, sampling was modified to also include these areas. The umbilical cord was inspected and representative specimens were collected for histopathology if lesions were present.
Histopathology
Specimens of the lung, heart, liver, spleen, kidney, adrenal gland, thymus, skeletal muscle (Musculus semitendinosus), cerebrum, brain stem, cerebellum, umbilical cord, and allantochorion were placed in 10% neutral buffered formalin.
Microbial Culture
Specimens of lung, liver, and allantochorion, as well as a sample of stomach content were collected at necropsy. the samples were submitted for microbial culture after storage overnight at 5°C .
- References
1. American Association of Laboratory Animal Science. Assistant Laboratory Animal Technician Training Manual. (Memphis, TN: Drumwright and Co, 2012).
2. Bergvall, K. (2005) Advances in acquisition,identication and treatment of equine ectoparasites. Clinical Techniques in Equine Practice, 4, 296–301.
3. Beale B. Arthropathies. In: Bloomberg M.S., Dee J.F., Taylor R.A., editors. Canine sports medicine and surgery. 1st edition. WB Saunders; Philadelphia: 1998. pp. 210–222.
4. Brownlow, M.A., Hutchins, D.R. & Johnston, K.G.(1981) Reference values for equine peritoneal fluid.
5. Collatos, C., Barton, M.H., Prasse, K.W. & Moore, J.N. (1995) Intravascular and peritoneal coagulation and fibrinolysis in horses with acute gastrointestinal tract diseases. Journal of the American Veterinary Medical Association, 207, 465–470.
6. Clark, C.H. & Woodley, C.H. (1959) The absorption of red blood cells after parenteral injection at various sites. American Journal of Veterinary Research, 20, 1062–1066.
7. Di Terlizzi R, Platt SR. The function, composition and analysis of cerebrospinal fluid in companion animals: Part II— Analysis. Vet J 2009;180:18.
8. Donovan, J., and Brown, P. Removal of blood from laboratory mammals and birds: First report of the BVA/FRAME/RSPCA/UFAW Joint working group on refinement. Lab Animal 27, 1-22. (1993) Equine Veterinary Journal, 13, 127–130.
9. Evans, A.G. and Stannard, A.A. (1986)Diagnostic approach to equine skin disease.Compendium Equine, 8, 652–660. Littlewood, J.D. (1997) Diagnostic procedures in equine skin disease. Equine Veterinary Education, 3, 174–176.
10. Furr M. Cerebrospinal fluid and the blood brain barrier. In: Equine neurology. Ames, IA: John Wiley & Sons; 2015, 30–34.
11. Johnston S. Joint anatomy, physiology, and pathobiology. Vet Clin North Am Small Anim Pract. 1997;27:699–734.
12. Johnson PJ, Contanstinescu GM. Collection of cerebrospinal fluid in horses. Equine Vet Educ 2000;12:7–12.
13. Marchevsky A., Read R. Bacterial septic arthritis in 19 dogs. Aust Vet J. 1999;77:233–237.
14. Pedersen N., Weisner K., Castles J. Noninfectious canine arthritis: the inflammatory, nonerosive arthritides. JAVMA. 1976;169:304–310
15. Hawk, C.T., Leary, S.T., and Morris, T.H. Formulary for Laboratory Animals (3rd ed.). (Ames, Iowa:Blackwell Publishing, 2005)
16. McCurnin, D., and Bassert, J. Clinical Textbook for Veterinary Technicians (5th ed.). (Philadelphia, PA: Saunders. 2002).
17. Murase H, Miyazawa M, Harada T, Ozawa M, Sato F, Hada T. Aborted fetal sizes of thoroughbred horses in Hidaka, Japan, between 2005 and 2015. J Equine Sci. (2017) 28:47–53. doi: 10.1294/jes.28.47.
18. Pascoe, R.R. and Knottenbelt, D.C. (1999)Manual of Equine Dermatology, W.B.Saunders, London.
19. Scott, D.W. and Miller, W.H. (2011) Equine Dermatology, 2nd edn, W.B. Saunders,St. Louis.
20. Sweeney CR, Russell GE. Differences in total protein concentration, nucleated cell count, and red blood cell count among sequential samples of cerebrospinal fluid from horses. J Am Vet Med Assoc 2000;217(1):54–56.
21. Van Hoogmoed, L., Rodger, L.D., Spier, S.J., Gardner, I.A., Yarbrough, T.B. & Snyder, J.R. (1999) Evaluation of peritoneal fluid pH, glucose concentration, and lactate dehydrogenase activity for detection of septic peritonitis in horses. Journal of the American Veterinary Medical Association, 214, 1032–1036.
22. Walesby, H.A. and Blackmer, J.M. How to Use the Transverse Facial Venous Sinus as an Alternative Location for Blood Collection in the Horse. In: (Ed.), 49th Annual Convention of the American Association of Equine Practitioners, 2003 - New Orleans, LA, USA. Ithaca: International Veterinary Information Service (www.ivis.org), 2003
23. Reed SM, Furr M, Howe DK, et al. Equine protozoal myeloencephalitis:An updated consensus statement with a focus on parasite biology, diagnostics, treatment, and prevention. J Vet Intern Med 2016; 30: 493. 2.
24. Roach JM, Foote AK, Smith KC, Verheyen KL, de Mestre AM. Incidence and causes of pregnancy loss after day 70 of gestation in Thoroughbreds. Equine Vet J. (2020) 2020:1–8. doi: 10.1111/evj.13386.
25. Weimann, C.D., Thoefner, M.B. & Jensen, A.L. (2002) Spectrophotometric assessment of peritoneal fluid haemoglobin in colic horses: An aid to selecting medical vs. surgical treatment. Equine Veterinary Journal, 34, 523–527.
26. Willard M. Fluid accumulation disorders. In: Willard M., Tvedten H., Turnwald G., editors. Small animal clinical diagnosis by laboratory methods. 1st edition. WB Saunders; Philadelphia: 1989. pp. 229–242.