BLADDER CANCER
| Site: | EHC | Egyptian Health Council |
| Course: | Oncology and Hematological Oncology Guidelines |
| Book: | BLADDER CANCER |
| Printed by: | Guest user |
| Date: | Monday, 23 December 2024, 9:19 PM |
Description
"last update: 21 Nov. 2024"
- Acknowledgement
➡️ We would like to acknowledge the Oncology Committee of Egyptian health council (EHC) Guidelines and Genito-urinary Cancer Scientific Committee, for adapting this Guidelines.
➡️ Chair of the Committee of Egyptian health council Guidelines: Prof Hussein Khaled.
➡️ The Oncology Committee Members: Ebtesam Saad Eldin, Ehab Khalil, Emad Hamada, Fouad Abuotaleb, Hesham Elghazaly, Hesham Tawfik, Khaled Abdelkarim, Lobna ezz Elarab, Mary Gamal, Mohamed Abdel Mooti, Mohamed Gamil, Nervana Hussein, Ola Khorshid, Omar Sherif Omar, Rasha Fahmi, Rasha Shaltout, Samir Shehata, Yousri Wasef and Yousri Rostom.
➡️ The Genito-urinary Cancer Scientific Committee Members: Ehab Khalil , Emad Hamada, Hatem Abul Kasem, Hassan Abol-Enein,, Hesham Tawfik, , Amr Elsawy, Ahmed Moein, Adel Atta, and Nervana Hussein
- Abbreviations
BCG, bacillus Calmette-Guerin.
ChT, chemotherapy.
CIS, carcinoma in situ.
EBRT, external beam radiotherapy.
EORTC, European Organization for Research and Treatment of Cancer.
G, grade.
HG UC, high grade urothelial carcinoma.
LG, low grade.
MIBC, muscle-invasive bladder cancer
NMIBC, non-muscle-invasive bladder cancer.
PLND, pelvic lymph node dissection
PS, performance status
PUNLMP, papillary urothelial neoplasm of low malignant potential.
RC, radical cystectomy.
TCGA, The Cancer Genome Atlas
TURBT, transurethral resection of the bladder tumor.
UTUC, Upper Tract Urothelial Carcinoma
- Executive Summary
|
Recommendations |
Strength of the recommendation |
|
DIAGNOSIS AND PATHOLOGY/MOLECULAR BIOLOGY |
|
|
Hematuria is the most common presenting symptom in bladder cancer and should in all cases be investigated |
Strong |
|
The diagnosis of bladder cancer is based on cystoscopic examination of the bladder and histological evaluation of tissue obtained either with cold-cup biopsy or TURBT, where complete resection of all tumor tissue should be achieved whenever possible and muscle tissue should be included in the biopsies, except when a Ta/LG is expected |
Strong |
|
Cross-sectional upper tract imaging (CT/MRI urography) is recommended to screen for synchronous UTUC, in cases of HG bladder cancer |
Conditional |
|
Pathological diagnosis should be made according to latest WHO classification |
Strong |
|
In addition to stage and grade, presence and percentage of variant histology, lymphovascular invasion and presence of muscularis propria should be reported |
Strong |
|
Urine cytology can facilitate the diagnosis of HG UC but cannot be used as the primary method of histological diagnosis |
Conditional |
|
STAGING AND RISK ASSESSMENT
|
|
|
Staging of NMIBC
|
|
|
Patients with NMIBC should be classified into four risk categories based on tumor characteristics (low , intermediate , high and very-high-risk) as shown in table 1.
|
Strong |
|
Regional and distant staging of MIBC
|
|
|
In patients with invasive disease (>T1), regional and distant staging should be carried out with further imaging studies such as contrast-enhanced CT of chest-abdomen-pelvis or MRI of abdomen/pelvis combined with chest CT |
Strong |
|
FDG-PET-CT may aid in the detection of LN and distant metastases, and in cases with impaired renal function. |
Conditional |
|
MANAGEMENT OF LOCAL/LOCOREGIONAL DISEASE |
|
|
|
|
|
In patients with low-risk NMIBC and those with small papillary recurrences, detected >1 year after the previous tumor, single, immediate, intravesical chemotherapy instillation, such as mitomycin C, or gemcitabine is recommended, in combination with continued cystoscopic surveillance |
Strong |
|
In patients with intermediate-risk NMIBC, additional courses of intravesical therapy are recommended, and is consisting of either instillations of Chemotherapy for a maximum of 1 year, or 12 months of BCG instillation therapy with six BCG instillations at weekly intervals, followed by three BCG instillations each at 3, 6 and 12 months |
Strong |
|
In patients with high-risk NMIBC, full dose intravesical BCG for 1-3 years (at least 1 year) is recommended with induction as previously mentioned for 6 weeks followed by instillations at 3, 6, 12, 18, 24, 30 and 36 months |
Strong |
|
Planned cystoscopic surveillance per high risk NMIBC schedule should be performed. |
Strong |
|
In case of very high risk or BCG unresponsive, radical cystectomy could be offered |
Conditional |
|
Treatment of MIBC
|
|
|
RC with standard PLND and protection of small intestine is the standard treatment of MIBC T2-T4a, N0 M0. |
Strong |
|
Patients with radiological suspicious node-positive disease (cN1) should be considered for preoperative platinum-based chemotherapy, however surgery can be offered in selected cases (e.g. unfit for systemic therapy, patient preference) |
Strong |
|
Organ-preservation therapy with radiotherapy, as part of multimodal schema for MIBC, is a reasonable option for patients with solitary tumors <7cm with no or unilateral hydronephrosis, and no extensive carcinoma in situ, also for patients seeking an alternative to RC and those who are medically unfit for surgery |
Conditional |
|
Contemporary organ-preservation protocols should utilize tri-modality combination of TURBT, radiotherapy and chemotherapy |
Strong |
|
Strong |
|
|
Three to four cycles of cisplatin-based neoadjuvant chemotherapy should be given for MIBC |
Strong |
|
The use of adjuvant cisplatin-based Chemotherapy in patients with pathologic T3, T4, N+ after cystectomy who did not receive neoadjuvant therapy should be considered |
Strong |
|
ddMVAC with growth factor support is the preferred regimen in the neoadjuvant setting , however Gemcitabine and cisplatin is a reasonable alternative |
Strong |
|
Carboplatin should not be substituted for cisplatin in the perioperative setting |
Strong |
|
For patients who are not candidates for cisplatin , there are no data to support a recommendation for perioperative chemotherapy |
Strong |
|
Bilateral pelvic lymphadenectomy should include removal of a minimum, the external and internal iliac and obturator lymph nodes. |
Strong |
|
Indications of Adjuvant radiotherapy after cystectomy 1- P T3 / T4 MIBC 2- Pathologically node positive 3- Positive margins
|
Strong |
|
Postoperative adjuvant RT
Postoperative adjuvant RT : Treatment field should encompass areas at risk for harboring residual microscopic disease based on pathologic findings at resection and include resection bed, and lymph nodes. Areas at risk for harboring residual microscopic disease should receive 45–50.4 Gy EBRT. Involved resection margins and areas of extranodal extension should be boosted to 54–60 Gy if feasible based on normal tissue constraints. Areas of gross residual disease should be boosted to 66–70 Gy, if feasible based on normal tissue constraints. Concurrent chemotherapy with regimens used for bladder cancer can be considered for added tumor cytotoxicity |
Strong |
|
Treatment of advanced/metastatic disease |
|
|
First line systemic therapy |
|
|
For Cisplatin eligible patients, gemcitabine and cisplatin or dd-MVAC (with growth factor support) regimens should be used |
Strong |
|
For Cisplatin ineligible patients, gemcitabine and carboplatin regimens should be used |
Strong |
|
Second and subsequent lines of therapy |
|
|
Patients with good PS Second line of therapy should include either single agents as gemcitabine, paclitaxel or docetaxel, or combination regimens as Gemcitabine and paclitaxel, or Ifosfamide, doxorubicin, and gemcitabine or other combinations |
Strong |
|
In patients with progression free survival > 12 months after platinum ( cisplatin or carboplatin ), consider re-treatment with platinum if the patient is still platinum eligible |
Conditional |
|
Palliative RT can be offered for palliation (bleeding, pain). |
Strong |
- Introduction
Bladder cancer is the second most common cancer in males and the third most common in the Egyptian population with more than 13 thousand newly diagnosed cases. (1) Moreover, it is also the third cause of cancer death in Egypt after hepatocellular carcinoma and breast cancer with estimated number of more than 7 thousand deaths in 2022.
➡️Purpose and scope
These guidelines will help to improve the quality of care for both NMIBL and MIBC patients via providing a uniform standard of care across the country to help in early diagnosis and treatment for bladder cancer, with less aggressive treatment options and improved clinical outcomes. These guidelines cover primary diagnosis, staging, treatment and follow-up of bladder cancer patients.
- Target audience
Clinicians who are involved in the care and treatment of patients with bladder cancer, including medical oncologists, radiation oncologists, clinical oncologists, onco- and uro-surgeons, interventional radiologists, radiologists and pathologists.
- Methodology
A comprehensive search for guidelines was undertaken to identify the most relevant guidelines to consider for adaptation. inclusion/exclusion criteria followed in the search and retrieval of guidelines to be adapted:
- Selecting only evidence-based guidelines (guidelines must include a report on systematic literature searches and explicit links between individual recommendations and their supporting evidence). - Selecting only national and/or international guidelines.
- Specific range of dates for publication (using Guidelines published or updated 2015 and later).
- Selecting peer reviewed publications only.
- Selecting guidelines written in English language.
- Excluding guidelines written by a single author not on behalf of an organization to be valid and comprehensive, a guideline ideally requires multidisciplinary input.
- Excluding guidelines published without references as the panel needs to know whether a thorough literature review was conducted and whether current evidence was used in the preparation of the recommendations.
All retrieved Guidelines were screened and appraised using AGREE II instrument (www.agreetrust.org) by at least two members. the panel decided a cutoff point or rank the guidelines (any guideline scoring above 50% on the rigor dimension was retained)
The NCCN, ESMO, NICE guidelines are the main sources used while formulating the national guidelines for bladder cancer (1-3).
- Evidence assessment
According to WHO handbook for Guidelines we used the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach to assess the quality of a body of evidence, develop and report recommendations. GRADE methods are used by WHO because these represent internationally agreed standards for making transparent recommendations. Detailed information on GRADE is available through the on the following sites:
. GRADE working group: http://www.gradeworkingroup.org
. GRADE online training modules: http://cebgrade.mcmaster.ca/
. GRADE profile software: http://ims.cochrane.org/revman/gradepro
Table 1: Quality of evidence in GRADE
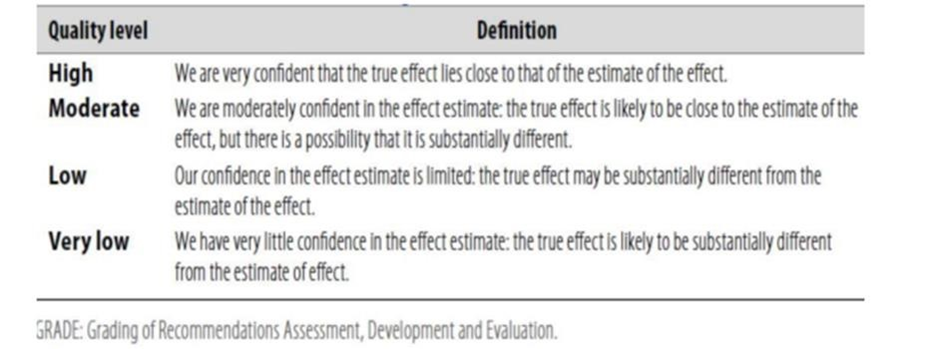
Table 2: Significance of the four levels of evidence

Table 3: Factors that determine how to upgrade or downgrade the quality of evidence.
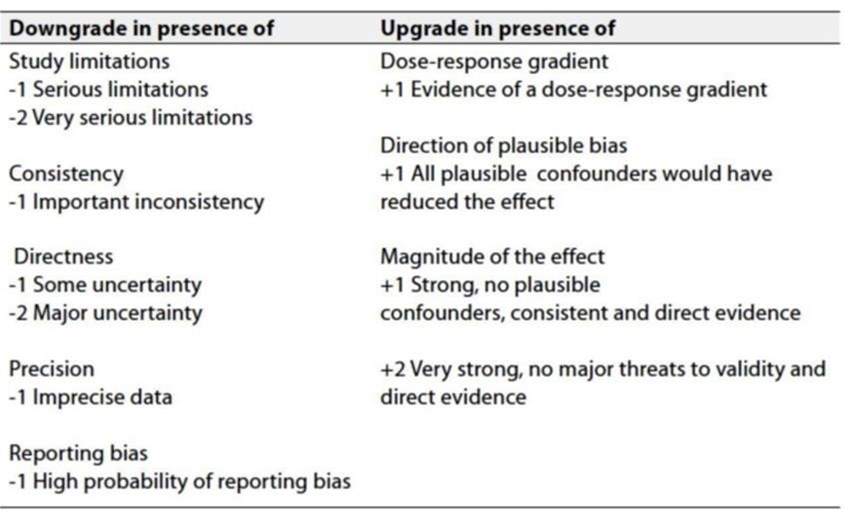
- The strength of the recommendation
The strength of a recommendation communicates the importance of adherence to the recommendation:
Strong recommendations: With strong recommendations, the guideline communicates the message that the desirable effects of adherence to the recommendation outweigh the undesirable effects. This means that in most situations the recommendation can be adopted as policy.
Conditional recommendations: These are made when there is greater uncertainty about the four factors above (Table 2) or if local adaptation must account for a greater variety in values and preferences, or when resource use makes the intervention suitable for some, but not for other locations. This means that there is a need for substantial debate and involvement of stakeholders before this recommendation can be adopted as policy.
When not to make recommendations; when there is lack of evidence on the effectiveness of an intervention, it may be appropriate not to make a recommendation.
- Recommendations
1. DIAGNOSIS AND PATHOLOGY/MOLECULAR BIOLOGY
· Hematuria is the most common presenting symptom in bladder cancer and should in all cases be investigated
Strong recommendation, High grade evidence (4).
· The diagnosis of bladder cancer is based on cystoscopic examination of the bladder and histological evaluation of tissue obtained either with cold-cup biopsy or TURBT. Complete resection of all tumour tissue should be achieved when possible. Muscle tissue should be included in the biopsies, except when a Ta/LG is expected
Strong recommendation, High grade evidence (4).
· Cross-sectional upper tract imaging (CT/MRI urography) is recommended to screen for synchronous UTUC, in cases of HG bladder cancer
Conditional recommendation, moderate grade evidence (5).
· Pathological diagnosis should be made according to latest WHO classification
Strong recommendation, moderate grade evidence (6).
· In addition to stage and grade, presence and percentage of variant histology, lymphovascular invasion and presence of muscularis propria should be reported
Strong recommendation, High grade evidence (6).
· Urine cytology can facilitate the diagnosis of HG UC but cannot be used as the primary method of histological diagnosis
Conditional recommendation, moderate grade evidence (7).
2. STAGING AND RISK ASSESSMENT
Staging of NMIBC
· Patients with NMIBC are classified into four risk categories based on tumor characteristics (low , intermediate , high and very-high-risk) as shown in table 1.
Strong recommendation, high grade evidence (9,10).
Regional and distant staging of MIBC
· In patients with invasive disease (>T1), regional and distant staging should be carried out with further imaging studies such as contrast-enhanced CT of chest-abdomen-pelvis or MRI of abdomen/pelvis combined with chest CT.
Strong recommendation, moderate grade evidence (11).
· FDG-PET-CT may aid in the detection of LN and distant metastases, and in cases with impaired renal function.
Conditional recommendation, low grade evidence (12).
3. MANAGEMENT OF LOCAL/LOCOREGIONAL DISEASE
Treatment of NMIBC
· In patients with low-risk NMIBC and those with small papillary recurrences, detected >1 year after the previous tumor, single, immediate, intravesical chemotherapy instillation, such as mitomycin C or gemcitabine, is recommended, in combination with continued cystoscopic surveillance.
Strong recommendation, high grade evidence (13,14)
· In patients with intermediate-risk NMIBC, additional courses of intravesical therapy are recommended, and is consisting of either instillations of Chemotherapy for a maximum of 1 year, or 12 months of BCG instillation therapy with six BCG instillations at weekly intervals, followed by three BCG instillations each at 3, 6 and 12 months.
Strong recommendation, high grade evidence (15).
· In patients with high-risk NMIBC, full dose intravesical BCG for 1-3 years (at least 1 year) is recommended with induction as previously mentioned for 6 weeks followed by instillations at 3, 6, 12, 18, 24, 30 and 36 months.
Strong recommendation, high grade evidence (16).
· Planned cystoscopic surveillance per high risk NMIBC schedule should be performed.
Strong recommendation, high grade evidence (17)
· In case of very high risk or BCG unresponsive, radical cystectomy could be offered.
Conditional recommendation, moderate grade evidence (17).
Treatment of MIBC
· RC with standard PLND and protection of small intestine is the standard treatment of MIBC T2-T4a, N0 M0.
Strong recommendation, high grade evidence (18).
· Patients with radiological suspicious node-positive disease (cN1) should be considered for preoperative platinum-based chemotherapy, however surgery can be offered in selected cases (e.g. unfit for systemic therapy, patient preference)
Strong recommendation, moderate grade evidence (19-21).
· Organ-preservation therapy with radiotherapy, as part of multimodal schema for MIBC, is a reasonable option for patients with solitary tumors <7cm with no or unilateral hydronephrosis, and no extensive carcinoma in situ, also for patients seeking an alternative to RC and those who are medically unfit for surgery
Conditional recommendation, moderate grade evidence (22).
· Contemporary organ-preservation protocols should utilize tri-modality combination of TURBT, radiotherapy and chemotherapy.
Strong recommendation, moderate grade evidence (23).
· Following completion of bladder preserving therapy, clinicians should perform regular surveillance with computed tomography (CT) scans, cystoscopy, and urine cytology.
Strong recommendation, moderate grade evidence (23).
· Three to four cycles of cisplatin-based neoadjuvant chemotherapy should be given to MIBC.
Strong recommendation, high grade evidence (24-28).
· The use of adjuvant cisplatin-based Chemotherapy in patients with pathological T3/T4/N+ who did not receive neoadjuvant therapy should be considered.
Strong recommendation, moderate grade evidence (27).
· ddMVAC with growth factor support is the preferred regimen in the neoadjuvant setting, however Gemcitabine and cisplatin is a reasonable alternative
Strong recommendation, moderate grade evidence (27).
· Carboplatin should not be substituted for cisplatin in the perioperative setting
Strong recommendation, moderate grade evidence (29)
· For patients who are not candidates for cisplatin , there are no data to support a recommendation for perioperative chemotherapy
Strong recommendation, moderate grade evidence (29)
· Standard radical cystectomy with curative intent need to obtain negative margins and should include removal of the bladder, prostate, and seminal vesicles in males; bladder in females and should consider removal of adjacent reproductive organs based on individual disease characteristics. Bilateral pelvic lymphadenectomy should include removal of a minimum, the external and internal iliac and obturator lymph nodes.
Strong recommendation, moderate grade evidence (30)
· Indications of adjuvant radiotherapy after cystectomy:
-P T3 / T4 MIBC
-Pathologically node positive
-Positive margins
Strong recommendation, moderate grade evidence (31)
· Postoperative adjuvant RT : Treatment field should encompass areas at risk for harboring residual microscopic disease based on pathologic findings at resection and include resection bed, and lymph nodes. Areas at risk for harboring residual microscopic disease should receive 45–50.4 Gy EBRT. Involved resection margins and areas of extranodal extension should be boosted to 54–60 Gy if feasible based on normal tissue constraints. Areas of gross residual disease should be boosted to 66–70 Gy, if feasible based on normal tissue constraints. Concurrent chemotherapy with regimens used for bladder cancer can be considered for added tumor cytotoxicity
Strong recommendation, moderate grade evidence (32)
4- Treatment of advanced/metastatic disease
First line systemic therapy
· For Cisplatin eligible patients, gemcitabine and cisplatin or dd-MVAC (with growth factor support) regimens should be used.
Strong recommendation, high grade evidence (33-38).
· For Cisplatin ineligible patients, gemcitabine and carboplatin regimens should be used.
Strong recommendation, high grade evidence (39).
Second and subsequent lines of therapy
· Patients with good PS
Second and subsequent lines of therapy should include either single agents as gemcitabine, paclitaxel, or docetaxel, or combination regimens as Gemcitabine and paclitaxel, or Ifosfamide, doxorubicin, and gemcitabine or other combinations
Strong recommendation, moderate grade evidence (40,41).
· In patients with progression free survival > 12 months after platinum (cisplatin or carboplatin), consider re-treatment with platinum if the patient is still platinum eligible
Conditional recommendation, moderate grade evidence (40,41).
• Palliative RT can be offered for palliation (bleeding, pain).
Strong recommendation, moderate grade evidence (42)
➡️ Clinical indicators for monitoring
See Annex 4
➡️ Research Gaps
• Evaluation of real world data on the use on new targeted and immune-therapeutic agents in bladder cancer in Egypt.
• Cost effective analysis of new therapeutic agents in Egypt.
• Define the molecular biologic profiles of our patients with mixed and variant tumors.
➡️ Update of the guideline
• This guideline will be updated whenever there is new evidence.
- Annexes
|
Annex 1. Risk group stratification of patients with NMIBC and treatment recommendations (1) |
|
|
Risk group stratification |
Characteristics |
|
Low-risk tumors |
Primary, solitary, Ta G1 (PUNLMP, LG), <3 cm, no CIS |
|
Intermediate-risk tumors |
All tumors not defined in the two adjacent categories (between the category of low and high risk) |
|
High-risk tumors |
Any of the following: _ T1 tumour _ HG tumour _ CIS _ Multiple, recurrent and large (>3 cm) Ta G1-G2/LG tumours (all features must be present |
|
Subgroup of highest-risk tumors |
_ T1 G3/HG associated with concurrent bladder CIS _ Multiple and/or large T1 G3/HG and/or recurrent T1 G3/HG, T1 G3/HG with CIS in the prostatic urethra _ Some forms of variant histology of urothelial carcinoma, lymphovascular invasion |
|
Annex 2. American Joint Committee on Cancer (AJCC) TNM Staging System for Bladder Cancer 8th ed., 2017), (1). T Primary Tumor TX Primary tumor cannot be assessed T0 No evidence of primary tumor Ta Noninvasive papillary carcinoma Tis Urothelial carcinoma in situ: “flat tumor” T1 Tumor invades lamina propria (subepithelial connective tissue) T2 Tumor invades muscularis propria pT2a Tumor invades superficial muscularis propria (inner half) pT2b Tumor invades deep muscularis propria (outer half) T3 Tumor invades perivesical tissue pT3a Microscopically pT3b Macroscopically (extravesical mass) T4 Extravesical tumor directly invades any of the following: prostatic stroma, seminal vesicles, uterus, vagina, pelvic wall, abdominal wall T4a Extravesical tumor invades prostatic stroma, seminal vesicles, uterus, vagina T4b Extravesical tumor invades pelvic wall, abdominal wall N Regional Lymph Nodes NX Lymph nodes cannot be assessed N0 No lymph node metastasis N1 Single regional lymph node metastasis in the true pelvis (perivesical, obturator, internal and external iliac, or sacral lymph node) N2 Multiple regional lymph node metastasis in the true pelvis (perivesical, obturator, internal and external iliac, or sacral lymph node metastasis) N3 Lymph node metastasis to the common iliac lymph nodes M Distant Metastasis M0 No distant metastasis M1 Distant metastasis M1a Distant metastasis limited to lymph nodes beyond the common iliacs M1b Non-lymph-node distant metastases
|
|
Annex 3. AJCC Prognostic Groups (T N M), (1). Stage 0a Ta N0 M0 Stage 0is Tis N0 M0 Stage I T1 N0 M0 Stage II T2a N0 M0 T2b N0 M0 Stage IIIA T3a N0 M0 T3b N0 M0 T4a N0 M0 T1-T4a N1 M0 Stage IIIB T1-T4a N2,N3 M0 Stage IVA T4b Any N M0 Any T Any N M1a Stage IVB Any T Any N M1b |
Annex 4. Follow up and patient monitoring during treatment
· No single follow-up plan is appropriate for all patients. The follow-up tables are to provide guidance, and should be modified for the individual patient based on sites
of disease, biology of disease, and length of time on treatment.
· Reassessment of disease activity should be performed in patients with new or worsening signs or symptoms of disease, regardless of the time interval from previous studies.
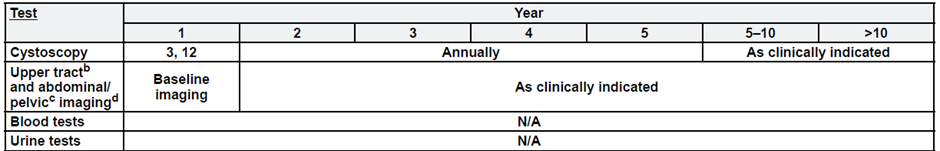
Low-Risk, Non-Muscle Invasive Bladder Cancer
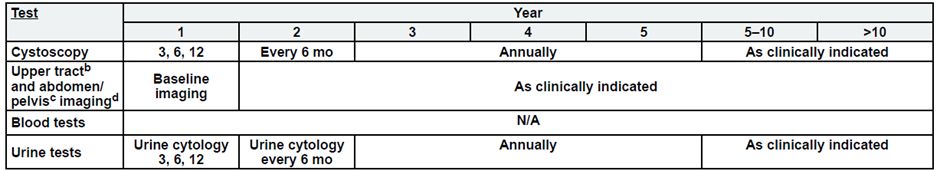
Intermediate Risk, Non-Muscle Invasive Bladder Cancer
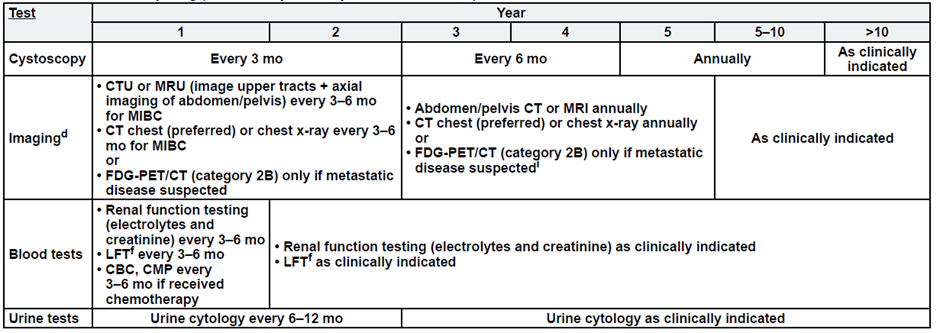
High-Risk, Non-Muscle Invasive Bladder Cancer
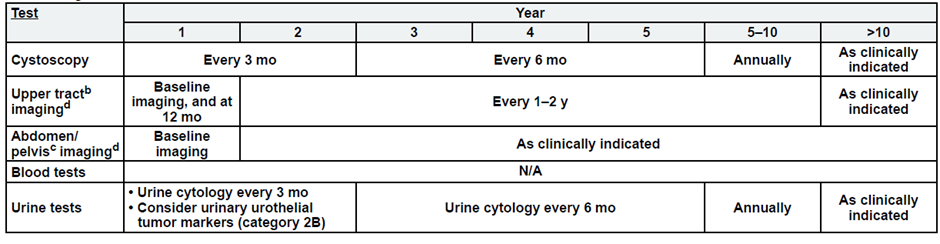
Post-Cystectomy Non-Muscle Invasive Bladder Cancer
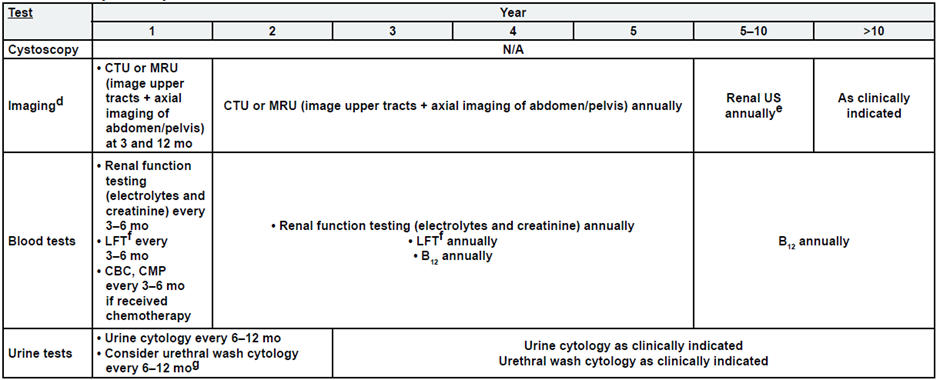
Post-Cystectomy Muscle Invasive Bladder Cancer
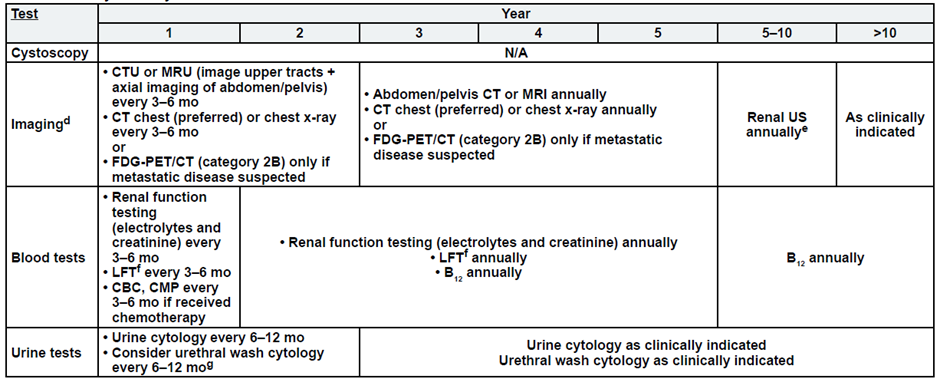
Post-Bladder Sparing
Metastatic Disease:
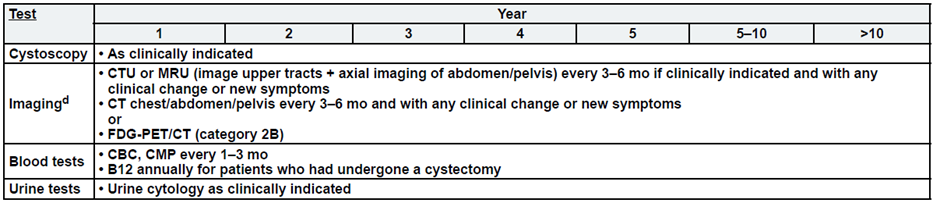
a. See risk classification
b. Upper tract imaging includes CTU, MRU, intravenous pyelogram (IVP), retrograde pyelography,
or ureteroscop.
c. Abdominal/pelvic imaging includes CT or MRI.
d. See imaging.
- References
- https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1417
- https://www.esmo.org/guidelines/guidelines-by-topic/esmo-clinical-practice-guidelines-genitourinary-cancers/clinical-practice-guideline-bladder-cancer
- https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-guidelines
- Ahmadi H, Duddalwar V, Daneshmand S., Diagnosis and Staging of Bladder Cancer., Hematol Oncol Clin North Am. 2021 Jun;35(3):531-541
- Trinh TW, Glazer DI, Sadow CA, et al. Bladder cancer diagnosis with CT urography: test characteristics and reasons for false-positive and false-negative results. Abdom Radiol (NY). 2018;43(3):663-671.
- Humphrey PA, Moch H, Cubilla AL, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur Urol. 2016;70(1):106-119.
- Yafi FA, Brimo F, Steinberg J, et al. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol Oncol. 2015;33(2):66.e25-66.e31.
- Loriot Y, Necchi A, Park SH, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381(4):338-348.
- Cambier S, Sylvester RJ, Collette L, et al. EORTC Nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance bacillus Calmette-Guerin. Eur Urol. 2016;69(1):60-69.
- 13. Rieken M, Xylinas E, Kluth L, et al. Long-term cancer-specific outcomes of TaG1 urothelial carcinoma of the bladder. Eur Urol. 2014;65(1):201-209.
- Siegel C. Bladder cancer: analysis of multi-detector row helical CT enhancement pattern and accuracy in tumor detection and perivesical staging. J Urol. 2005;174(4 Pt 1):1250-1251.
- Swinnen G, Maes A, Pottel H, et al. FDG-PET/CT for the preoperative lymph node staging of invasive bladder cancer. Eur Urol. 2010;57(4): 641-647.
- Rieken M, Xylinas E, Kluth L, et al. Long-term cancer-specific outcomes of TaG1 urothelial carcinoma of the bladder. Eur Urol.2014;65(1):201-209.
- Sylvester RJ, Oosterlinck W, Holmang S, et al. Systematic review and Individual patient data meta-analysis of randomized trials comparing a single immediate instillation of hemotherapy after transurethral resection with transurethral resection alone in patients with stage pTa-pT1 urothelial carcinoma of the bladder: which patients benefit from the instillation? Eur Urol. 2016;69(2):231-244.
- Malmstrom PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. Eur Urol. 009;56(2):247-256.
- Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guerin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013;63(3):462-472.
- Kamat AM, Sylvester RJ, Bohle A, et al. Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: recommendations from the International Bladder Cancer Group. J Clin Oncol. 2016;34(16):1935-1944.
- Gakis G, Black PC, Bochner BH, et al. Systematic review on the fate of the remnant urothelium after radical cystectomy. Eur Urol.2017;71(4):545-557.
- Afferi L, Zamboni S, Karnes RJ, et al. The impact of treatment modality on survival in patients with clinical node-positive bladder cancer: results from a multicenter collaboration. World J Urol. 2021;39(2):443-451.
- Abufaraj M, Dalbagni G, Daneshmand S, et al. The role of surgery in metastatic bladder cancer: a systematic review. Eur Urol. 2018;73(4):543-557.
- Abufaraj M, Al-Ani A, AlQudah A, et al. Surgical intervention in patients with urothelial carcinoma of the bladder and lymph node metastasis. Curr Opin Urol. 2021;31(3):220-225.
- Coppin CM, Gospodarowicz MK, James K, et al. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1996;14(11):2901-2907.
- Ploussard G, Daneshmand S, Efstathiou JA, et al. Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: a systematic review. Eur Urol. 2014;66(1):120-137.
- Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48(2):202-205. discussion 205-206.
- Zargar H, Shah JB, van Rhijn BW, et al. Neoadjuvant dose dense MVAC versus gemcitabine and cisplatin in patients with cT3-4aN0M0 bladder cancer treated with radical cystectomy. J Urol. 2018;199(6): 1452-1458.
- Galsky MD, Pal SK, Chowdhury S, et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015;121(15):2586-2593.
- Pfister C, Gravis G, Flechon A, et al. Randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin as perioperative chemotherapy for patients with muscle-invasive bladder cancer. Analysis of the GETUG/AFU V05 VESPER Trial secondary endpoints: chemotherapy toxicity and pathological responses. Eur Urol. 2021;79(2):214-221.
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859-866.
29. Dash A, Pettus JA, Herr HW, et al .A role for neoadjuvant gemcitabine plus cisplatin in Muscle invasive urothelial carcinoma of the bladder : a retrospective experience .Cancer 2008;113:2471-2477
30. Zlotta AR, Ballas LK, Niemierko A, et al. Radical cystectomy versus trimodality therapy for muscle invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. Lancet Oncol. 2023;24(6):669-681.
31. Baumann BC, He J, Hwang WT, et al. Validating a local failure risk stratification for use in prospective studies of adjuvant radiation therapy for bladder cancer. Int J Radiat Oncol Biol Phys 2018;95:703-706.
32. Baumann BC, Bosch WR, Bahl A, et al. Development and validation of consensus contouring guidelines for adjuvant radiation therapy for bladder cancer after Radical cystectomy. Int J Radiat Oncol Biol Phys 2018;96:78-86.
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068-3077.
- Loehrer PJ Sr, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1992;10(7):1066-1073.
- Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumors. Eur J Cancer. 2006;42(1):50-54.
- Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol. 2012;30(10):1107-1113.
- Rosenberg JE, Ballman KV, Halabi S, et al. CALGB 90601 (Alliance): randomized, double-blind, placebo-controlled phase III trial comparing gemcitabine and cisplatin with bevacizumab or placebo in patients with metastatic urothelial carcinoma. J Clin Oncol. 2019;37(suppl 15):4503.
- Rosenberg JE, Ballman KA, Halabi S, et al. Randomized phase III trial of gemcitabine and cisplatin with bevacizumab or placebo in patients with advanced urothelial carcinoma: results of CALGB 90601 (Alliance).J Clin Oncol. 2021;39(22):2486-2496.
- Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer “unfit” for Cisplatin-based chemotherapy. J Clin Oncol. 2011;29(17):2432-2438.
- Siefker-Radtke AO, Dinney CP, Shen Y, et al: A phase 2 clinical trial of sequential neoadjuvant chemotherapy with ifosfamide, doxorubicin, and gemcitabine followed by cisplatin, gemcitabine, and ifosfamide in locally advanced urothelial cancer: final results. Cancer 2013;119:540-547.
41. Albers P, Park SI, Niegisch G, et al. Randomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: short-term versus prolonged treatment [German Association of Urological Oncology (AUO) trial AB 20/99]. Ann Oncol 2011; 22:288-294.
42. Ali A, Song Y, Mehta S, et al . Palliative Radiotherapy Therapy in Bladder Cancer-Importance of Patient Selection. A Retrospective Multicenter Study .Int J Radiat Oncol Biol Phys 2019 ; 105:389-93.