Personal Protective Equipment
| Site: | EHC | Egyptian Health Council |
| Course: | Infection Prevention and Control Guidelines |
| Book: | Personal Protective Equipment |
| Printed by: | Guest user |
| Date: | Monday, 23 December 2024, 9:04 PM |
Description
"last update: 28 Oct 2024"
- Acknowledgements
We would like to acknowledge the Infection Control Guidelines Committee for developing these guidelines.
▪️ Head of IPC Guidelines Committee
Prof Dr Ghada Ismail (Professor of Clinical Pathology (Clinical Microbiology), Faculty of Medicine, Ain Shams University, Secretary of Supreme IPC Committee, SCUH, Member of WHO Global Guidelines Groups (GDG) for Infection Prevention)
▪️ Secretary of IPC Guidelines Committee
Prof Dr Walaa Abd El-Latif (Professor of Medical Microbiology and Immunology, Faculty of Medicine Ain Shams University, IPC Consultant)
▪️ Members of the Committee
- Prof Dr Amal Sayed (Deputy Manager of Environmental Affair, Infection Control Director, Cairo University Hospitals)
- Prof Dr Amani El-Kholy (Clinical Pathology Department (Microbiology), Faculty of Medicine, Cairo University, Infection Control Consultant)
- Dr Gehan Mohamed Fahmy (Professor clinical microbiology ASUSH consultant infection control, Board member of IFIC EMERO region)
- Prof Dr Hebatallah Gamal Rashed (Clinical Pathology Department (Microbiology), Faculty of Medicine, Assuit University, Infection Control Consultant)
- Dr Iman Afifi (Consultant Clinical Pathology (Microbiology) and IPC, Ain Shams University, Director IPC units of Ain Shams internal medicine and Geriatric hospitals
- Prof Dr Maha El Touny (Department of internal medicine. Faculty of Medicine, Ain Shams University. Infection Control Consultant)
- Prof Dr Nagwa Khamis (Emeritus Consultant Clinical Pathology (Microbiology) and IPC, ASU Director IPC Department and CEO Consultant IPC, CCHE-57357)
- Prof Dr Nesrine Fathi Hanafi (Professor in Medical Microbiology and Immunology Faculty of Medicine Alexandria University, Head of Infection Prevention and Control, Alexandria University Hospitals)
- Dr. Reham Lotfy Abdel Aziz (Environmental Health Director, EEAA, Hazardous Waste Consultant, WMRA, Ministry of Environment)
- Prof Dr Sherin ElMasry (Professor of Clinical Pathology, Ain Shams University, Chief Director of IPC ASU, Health Care Quality & Patient Safety Consultant)
- Dr Shimaa El-Garf (Coordinator): Clinical Pathology Specialist, Coordinator of HAI Surveillance and Audit Electronic System for University Hospitals, RLEUH- SCUH
- Glossary of Terms
- Airborne transmission: A
means of spreading infection when airborne droplet nuclei (small particle
residue of evaporated droplets ≤5 μm in size containing microorganisms that
remain suspended in air for long periods of time) are inhaled by the
susceptible host.
- Contact precautions:
Measures used to prevent and control infections that spread via direct contact
with the patient or indirectly from the patient’s immediate care environment
(including care equipment).
- Droplet transmission:
The spread of infection from one individual to another by droplets containing
infectious agents.
- Outbreak: When there are more disease cases
than what is usually expected for a given time, within a specific location, for
a target population.
- Pathogen: A pathogen is any organism
that causes disease. This includes bacteria, viruses, fungi, and parasites.
Pathogens are also known as infectious agents because they can cause infections
in their hosts.
- Personal Protective Equipment Executive Summary
➡️Personal protective
equipment (PPE) is equipment used to prevent or minimize exposure to hazards
such as:
· Biological hazards
· Chemical hazards
· Radiological
hazards
· Electrical hazards
· Mechanical hazards
· Etc.
➡️PPE protects
healthcare workers from mainly biological hazards which include:
· Person-to-person
contact
· Droplet spread
· Airborne
transmission
· Contaminated
objects (fomites)
➡️Risk assessment
· Assess the risk of
exposure to blood and body fluids, secretions/ excretions, splashes or sprays
or contaminated surfaces before any health care activity.
Select
the appropriate actions to reduce the risk of exposure to infectious agents.
|
Serial |
Recommendations |
|
1 |
Ensure proper selection and use of PPE based on the nature of the patient interaction and potential for exposure to blood, body fluids and/or infectious material: a. Wear gloves when it can be reasonably anticipated that contact with blood or other potentially infectious materials, mucous membranes, non-intact skin, potentially contaminated skin or contaminated equipment could occur. (Strong recommendation) b. Wear gown that is appropriate to the task to protect skin and prevent soiling of clothing during procedures and activities that could cause contact with blood, body fluids, secretions, or excretions (Strong recommendation) c. Use protective eyewear and a mask, or a face shield, to protect the mucous membranes of the eyes, nose and mouth during procedures and activities that could generate splashes or sprays of blood, body fluids, secretions and excretions. Select masks, goggles, face shields, and combinations of each according to the need anticipated by the task performed (Strong recommendation) d. Remove and discard PPE, other than respirators, upon completing a task before leaving the patient’s room or care area. If a respirator is used, it should be removed and discarded (or reprocessed if reusable) after leaving the patient room or care area and closing the door (Strong recommendation) e. Do not use the same gown or pair of gloves for care of more than one patient. Remove and discard disposable gloves upon completion of a task or when soiled during the process of care (Strong recommendation) f. Do not wash gloves for the purpose of reuse (Strong recommendation) |
|
2 |
Ensure that healthcare personnel have immediate access to and are trained and able to select, put on, remove, and dispose of PPE in a manner that protects themselves, the patient, and others (Strong recommendation) |
- Introduction
1.1.1 Personal Protective Equipment is
of critical importance in infection prevention and control (IPC), serving as a
primary defense against the transmission of infectious agents in healthcare and
other high-risk settings. PPE acts as a physical barrier that reduces direct
contact with infectious microorganisms such as bacteria, viruses, fungi, and
other pathogens, which can spread through airborne, droplet, or contact routes.
The use of PPE is essential to safeguarding healthcare workers, patients, and
the public from healthcare-associated infections (HAIs) and other infectious
diseases.
1.1.2 PPE used in infection prevention
protect specific body areas from infectious fluids, aerosols, or contaminated
surfaces. Its correct usage is essential to prevent HAIs, which remain a major
challenge in medical settings, leading to prolonged hospital stays, increased
healthcare costs, and higher patient morbidity and mortality.
1.1.3 The importance of PPE in infection
prevention and control was starkly highlighted during the coronavirus disease
2019 (COVID-19) pandemic, where widespread use of PPE such as N95 respirators,
surgical masks, and protective gowns was essential in preventing the spread of
the virus among healthcare professionals and the public. During such outbreaks,
the correct selection, use, and disposal of PPE become paramount to controlling
infection transmission.
1.1.4 The effectiveness of PPE in
infection control depends not only on the quality and design of the equipment
but also on adherence to strict protocols regarding donning (putting on) and
doffing (taking off), which minimize the risk of self-contamination. Equally
important is training healthcare personnel in the proper use of PPE, as
improper use or inconsistent application can lead to increased exposure to
pathogens.
- Scope and Purpose
1.1.1 The primary objectives of PPE are:
1.1.2 Types of PPE: The specific types of PPE used vary depending on the nature of the task or
procedure. Common examples include:
1.1.3 Risk Assessment and Selection
1.1.4 Training and Compliance
Compliance with PPE guidelines and policies is crucial
for ensuring the effectiveness of infection prevention and control measures.
Healthcare organizations should implement policies and procedures to promote
adherence to PPE guidelines and provide ongoing education and support to staff.
➡️Target Audience
1.2.1 Healthcare workers providing
patient care (All clinical staff –Nurse and Nurse Assistant.
1.2.2 IPC leads/focal persons and
teams in health care facilities.
1.2.3 Diagnostic imaging staff, laboratory
personnel, technicians, Pharmacist.
1.2.4 Auxiliary services
(Environmental services, central Reprocessing Sterilization Department Workers
(CSSD).
1.2.5 Mortuary staff.
1.2.6 Administrative staff.
1.2.7 Policy maker and health care manager & other
stakeholders, such as those responsible for health care quality improvement,
patient safety, health facility accreditation/regulation infectious disease
control and surveillance.
1.2.8 Patients and any one visitors
1.1.1.1 Protection of Healthcare Workers: PPE provides a physical barrier, minimizing the risk of exposure to pathogens and reducing the likelihood of healthcare-associated infections.
1.1.1.2 Safeguarding Patients: By preventing the transmission of infections from healthcare workers to patients, PPE contributes to patient safety and well-being.
1.1.1.3 Infection Prevention and Control: PPE plays a pivotal role in infection prevention and control strategies, helping to limit the spread of infectious diseases within healthcare settings.
1.1.2.1 Gloves: Disposable gloves are widely used to protect hands from contamination. Different types of gloves, such as nitrile, latex, or vinyl, are available based on the level of protection required.
1.1.2.2 Gowns: Gowns are worn to protect the body from exposure to blood and bodily fluids. They come in various styles, including surgical gowns, isolation gowns, and fluid-resistant gowns.
1.1.2.3 Masks: Masks are used to protect the nose, mouth, and face from airborne particles. Surgical masks, N95 respirators, and other types of masks are employed based on the specific risk assessment.
1.1.2.4 Eye Protection: Goggles, face shields, or safety glasses are used to protect the eyes from splashes of blood or other bodily fluids.
1.1.2.5 Aprons: Aprons are worn to protect the body from contamination during specific procedures, such as cleaning or handling contaminated materials.
The appropriate level of PPE is determined through a risk assessment, which evaluates the potential for exposure to biological hazards. Factors considered in risk assessment include the type of procedure, the patient's condition, and the likelihood of exposure to infectious agents. Based on the risk assessment, healthcare workers select the appropriate PPE to ensure adequate protection.
Proper training on the selection, use, and disposal of PPE is essential for healthcare workers. Training programs should cover topics such as:
1.1.4.1 PPE selection: Understanding the different types of PPE and when to use them.
1.1.4.2 Donning and doffing: Correct techniques for putting on and taking off PPE to minimize the risk of contamination.
1.1.4.3 Maintenance and disposal: Proper care and
disposal of PPE to prevent the spread of infection.
- Methodology
A comprehensive search for guidelines was undertaken to identify the most relevant guidelines to consider for adaptation.
Inclusion/ exclusion criteria followed in the search and retrieval of guidelines to be adapted:
▪️ Selecting only evidence-based guidelines (guideline must include a report on systematic literature searches and explicit links between individual recommendations and their supporting evidence)
▪️ Selecting only national and/or international guidelines
▪️ Specific range of dates for publication (using Guidelines published or updated in 2013 and later)
▪️ Selecting peer reviewed publications only
▪️ Selecting guidelines written in English language
▪️ Excluding guidelines written by a single author, not on behalf of an organization to be valid and comprehensive, a guideline ideally requires multidisciplinary input.
▪️ Excluding guidelines published without references as the panel needs to know whether a thorough literature review was conducted and whether current evidence was used in the preparation of the recommendations.
The following characteristics of the retrieved guidelines were summarized in:
▪️ Developing organisation/authors
▪️ Date of publication, posting, and release
▪️ Country/language of publication
▪️ Date of posting and/or release
▪️ Dates of the search used by the source guideline developers.
All retrieved Guidelines were screened and appraised using AGREE II instrument (www.agreetrust.org) by at least three members. The panel decided on a cut-off point or ranked the guidelines (any guideline scoring above 50% on the rigor dimension was retained). The committee decided to adapt from
1. WHO Guidelines on Hand Hygiene in Health Care 2009
2. Infection Prevention and Control (IPC) National Irish Clinical Guideline No. 30 May 2023 Vol 1
3. Guideline for Hand Hygiene in Health-Care Settings Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force 2002, updated March 2024
➡️Evidence assessment
According to WHO Handbook for Guidelines, we used the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach to assess the quality of a body of evidence, develop and report recommendations. GRADE methods are used by WHO because these represent internationally agreed standards for making transparent recommendations. Detailed GRADE information is available on the following sites:
▪️ GRADE working group: http://www.gradeworkingroup.org
▪️ GRADE online training modules: http://cebgrade.mcmaster.ca/
▪️ GRADE profile software: http://ims.cochrane.org/revman/gradepro
Table (1) Quality and Significance of the four levels of evidence in GRADE
|
Quality |
Definition |
Implications |
|
High |
The guideline development group is very confident that the true effect lies close to that of the estimate of the effect |
Further research is very unlikely to change confidence in the estimate effect |
|
Moderate |
The guideline development group is moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibly that it is substantially different |
Further research is likely to have an important impact on confidence in the estimate of the effect and may change the estimate |
|
Low |
Confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the true effect |
Further research is very likely to have an important on confidence in the estimate of effect and is unlikely to change the estimate |
|
Very low |
The group has very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect |
Any estimate of the effect is very uncertain |
Table (2) Factors that determine How
to upgrade or downgrade the quality of evidence.
|
Downgrade in presence of |
Upgrade in presence of |
|
Study limitations. 1- Serious limitations 2- Very serious limitations |
Dose- response gradient. +1 Evidence of a dose-response gradient |
|
Consistency 1- Important inconsistency |
Direction of plausible bias + All plausible confounders would have reduced the effect |
|
Directness 1- Some uncertainty 2- Major uncertainty |
Magnitude of the effect +1 Strong, no plausible Confounder, consistent and direct evidence |
|
Precision 1- Imprecise data |
+2 very strong, no major threats to validity and direct evidence |
|
Reporting bias 1- High probability of reporting bias |
|
The strength of the recommendations
The strength of a recommendation communicates the importance of adherence to the recommendation.
➡️ Strong recommendations
With strong recommendations, the guideline communicates the message that the desirable effects of adherence to the recommendation outweigh the undesirable effects. This means that in most situations the recommendation can be adopted as policy.
➡️ Conditional recommendations
These are made when there is greater uncertainty about the four factors above or if local adaptation has to account for a greater variety in values and preferences, or when resource use makes the intervention suitable for some, but not for other locations. This means that there is a need for substantial debate and involvement of stakeholders before this recommendation can be adopted as policy.
When not to make recommendations?
When there is lack of evidence on the effectiveness of an intervention, it may be appropriate not to make a recommendation.
- Recommendations
|
Serial |
Recommendations |
|
1 |
Ensure proper selection and use of PPE based on the nature of the patient interaction and potential for exposure to blood, body fluids and/or infectious material: a. Wear gloves when it can be reasonably anticipated that contact with blood or other potentially infectious materials, mucous membranes, non-intact skin, potentially contaminated skin or contaminated equipment could occur. (Strong recommendation, Moderate grade evidence) b. Wear a gown that is appropriate to the task to protect skin and prevent soiling of clothing during procedures and activities that could cause contact with blood, body fluids, secretions, or excretions (Strong recommendation, Moderate grade evidence) c. Use protective eyewear and a mask, or a face shield, to protect the mucous membranes of the eyes, nose and mouth during procedures and activities that could generate splashes or sprays of blood, body fluids, secretions and excretions. Select masks, goggles, face shields, and combinations of each according to the need anticipated by the task performed (Strong recommendation, Moderate grade evidence) d. Remove and discard PPE, other than respirators, upon completing a task before leaving the patient’s room or care area. If a respirator is used, it should be removed and discarded (or reprocessed if reusable) after leaving the patient room or care area and closing the door (Strong recommendation, Moderate grade evidence) e. Do not use the same gown or pair of gloves for care of more than one patient. Remove and discard disposable gloves upon completion of a task or when soiled during the process of care (Strong recommendation, Moderate grade evidence)f. Do not wash gloves for the purpose of reuse (Strong recommendation, Moderate grade evidence) |
1.1.1 Remarks
➡️ Health workers should
· Wear a gown to protect skin and prevent soiling
of clothing during activities that are likely to generate splashes or sprays of
blood, body fluids, secretions or excretions.
Note: if the gown is not fluid-resistant, and if splashing or
spraying is anticipated, a waterproof apron should be worn over the gown
· Remove the soiled gown as soon as possible and
perform hand hygiene.
· Wear a medical mask (also known as a surgical
or procedure mask) to protect mucous membranes of the nose and mouth against
splashes or sprays of body fluids, respiratory secretions and chemicals.
· Wear a medical mask to protect the patient
during aseptic procedures (e.g. During surgery or lumbar punctures).
· Wear either eye protection (eye visor, goggles)
or a face shield to protect mucous membranes of the eyes during activities that
are likely to generate splashes or sprays of blood, body fluids, secretions and
excretions.
· Ensure that goggles fit over and around the
eyes or personal prescription lenses.
·Ensure that a face shield covers the forehead,
extends below the chin, and wraps around the side of the face – note that face
shields are more comfortable to wear with eyeglasses.
· Wear a respirator (e.g. N95, ffp2, etc.) For protection
from inhalation of airborne particles (tiny particles that float in the air)
and/or when performing aerosol-generating procedures.
· Replace the mask or respirator if it is
damaged, soiled or wet, or if breathing becomes difficult.
·The wearer should properly be trained in its
safe use. Do a fit test before using a respirator for the first time, and
perform a seal check every time a respirator is used.
➡️Fit Testing
1. Fit testing is done to identify the style of respirator suitable
for each individual, and to ensure that it is worn correctly.
2.
If fit testing of all staff is not possible, fit testing should be
prioritized for those at greatest risk infection. These include the following:
· HCW’s most likely to be involved in performing AGPs, in particular
endotracheal intubation.
·HCW’s most likely to have the most frequent and or prolonged
exposure to airborne infection.
3. When to considered fit testing
-
At the commencement of employment
for employees with significant risk of exposure to infectious microorganisms
transmitted via the airborne route, for example risk is higher in an intensive
care unit, during physiotherapy and performing a procedure to induce sputum in
potentially infectious patients.
-
When a significant change in the Healthcare
workers (HCWs) facial characteristics lead to altering the facial seal of the
respirator, as in case of significant change in body weight or facial surgery.
➡️Fit Checking
To ensure that
the N95 or FFP2 respirator is properly applied i.e. properly sealed over the
bridge of the nose and mouth and that there are no gaps between the respirator
and face, health care workers must perform fit checks every time they put on
the respirator.
➡️Steps for fit checking includes:
1. Placement of the respirator on the face and place the headbands
over the head and at the base of the neck.
2.Compress the respirator to ensure proper sealing on the face,
cheeks and the bridge of the nose.
3.Check the positive pressure seal of the respirator by gently
exhaling. If air escapes, the respirator needs to be adjusted.
4.Checking the negative pressure seal of the respirator by gently
inhaling. If the respirator is not drawn in towards the face, HCW should
readjust the respirator and repeat process or check for defects in the
respirator.
➡️Special
Considerations when using an FFP2 respirator include
· If healthcare workers have facial hair, including a one to two-day
beard growth, or a long moustache adequate proper sealing cannot be guaranteed.
An alternative respirator such as a powered air purifying respirator should be
used.
·Do not touch respirator masks while being worn.
·Change the respirator masks when they become moist.
·
Do not reuse respirator masks after they have been removed.
·Do not dangle respirator masks around the neck.
· Perform hand hygiene upon touching or disposing of a used
respirator mask.
➡️Removal of N95
or FFP2 Respirator
Considerations when removing an FFP2 respirator to minimize the
risk of contamination to the user include:
1.
It should be removed outside the patient care area.
2. lean head forward, remove the respirator masks by the straps from
the back of the head forwards.
3. Disposed of in a closed receptacle.
4.Perform hand hygiene after removing the respirator.
1.1.1.1 Gloves
➡️Health workers should
- Wear gloves, according to Standard and Contact Precautions (as examples in the pyramid below), when contact with blood or other potentially infectious materials, mucous membranes, non-intact skin, potentially contaminated skin, or contaminated equipment could occur.
Wearing gloves doesn’t not a replace for hand hygiene.
·If your task requires gloves, perform hand hygiene prior to donning gloves, before touching the patient or the patient environment.
·Perform hand hygiene immediately after removing gloves.
Change gloves and perform hand hygiene during patient care, if
·gloves become damaged,
· gloves become visibly soiled with blood or body fluids following a task,
· moving from work on a soiled body site to a clean body site on the same patient or if another clinical indication for hand hygiene occurs.
Never wear the same pair of gloves in the care of more than one patient.
Carefully remove gloves to prevent hand contamination. (Annex.1)
Discard gloves after each task and clean your hands.
➡️The Glove Pyramid
Used to aid decision making on when to wear (and not wear) gloves. Gloves must be worn according to standard and contact precautions. The pyramid details some clinical examples in which gloves are not indicated, and others in which clean or sterile gloves are indicated. Hand hygiene should be performed when appropriate regardless of indications for glove use (Annex. 2)
1.1.1.2 Gown
1.1.1.3 Medical Masks
Health workers should
1.1.1.4 Eye protection
Health workers should
1.1.1.5 N95 or FFP2 Respirators
Health
workers should
|
Serial |
Recommendations |
|
2 |
Ensure that healthcare personnel have immediate access to and are trained and able to select, put on, remove, and dispose of PPE in a manner that protects themselves, the patient, and others (Strong recommendation, Moderate grade evidence) |
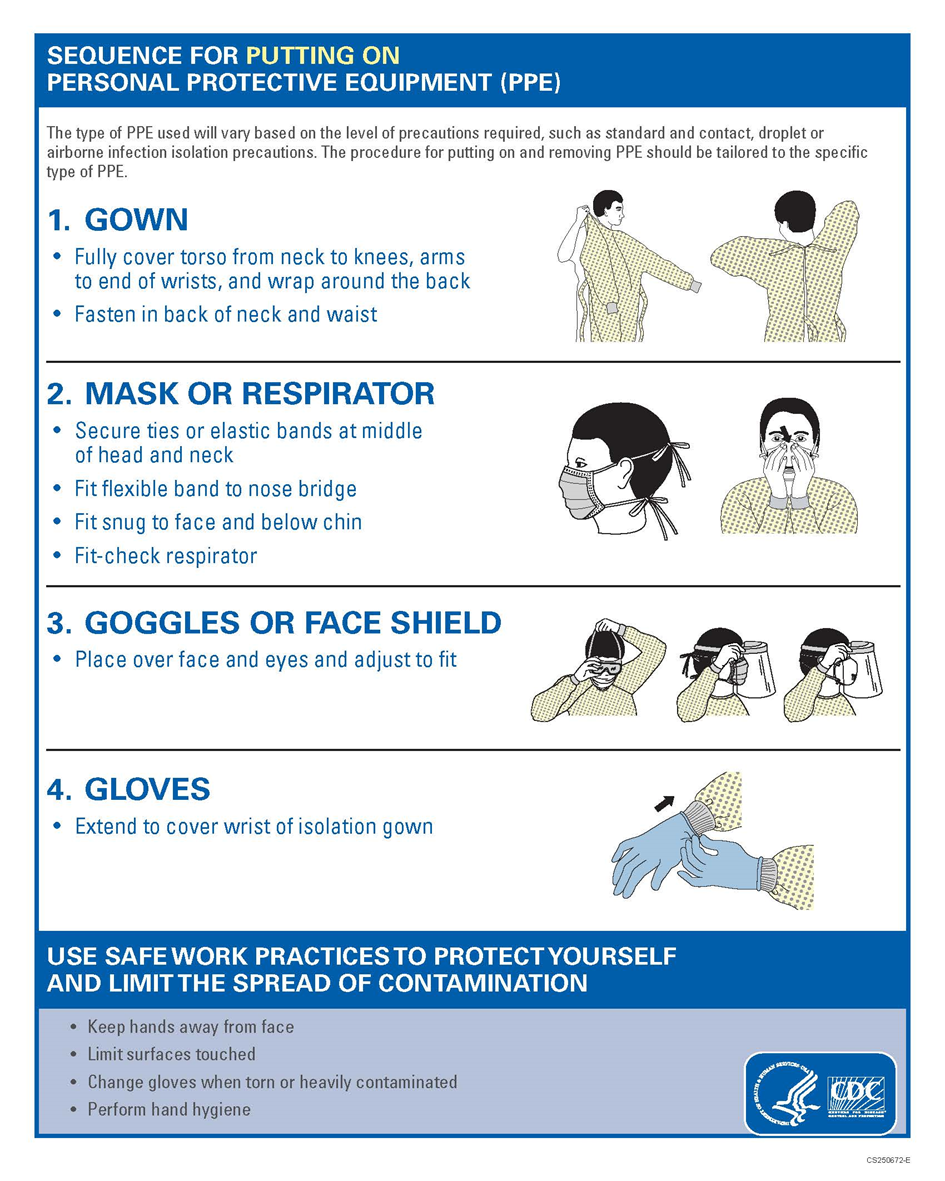
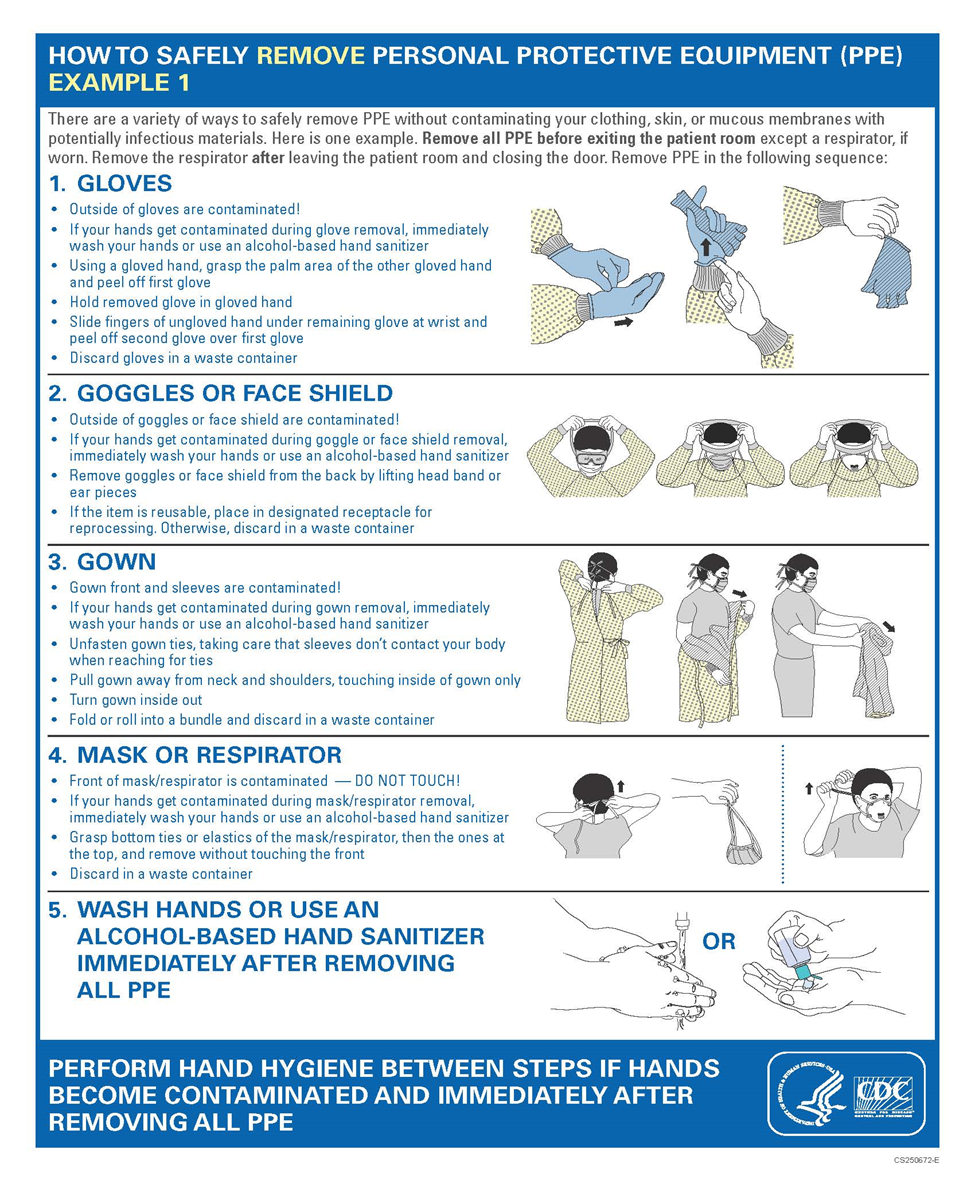
1.1.1 Remarks (Annex 3 Sequence for Putting and removing on PPE from CDC)
1.2 Indicators for Monitoring
To ensure the effectiveness of PPE utilization practices in hospitals and reduce the risk
of HAIs, specific indicators should be monitored regularly. These indicators provide measurable data to assess compliance, identify areas for improvement, and guide interventions. Here are some Key Performance Indicators (KPIs) that can be tailored
to suit different healthcare settings and contexts, ensuring thorough monitoring and evaluation of PPE usage and adherence to infection prevention and control protocols.
1.2.1 PPE Compliance Rate
· KPI
Definition: The percentage of healthcare workers correctly donning and doffing PPE according to infection prevention and control guidelines.
·Target: Each organization should set its target according to the strength of recommendation and
gap analysis.
· Calculation:
|
Compliance Rate = |
Number of Staff Using PPE Correctly |
X100 |
|
Total number of staff observed |
· Frequency: Weekly.
1.2.2 PPE Availability Rate
· KPI Definition: The percentage of time that PPE is available and accessible in required areas (e.g., patient care, high-risk zones).
· Target: Each organization should set its target according to the strength of recommendation and gap analysis.
· Calculation:
|
PPE Availability Rate = |
Number of days PPE was available in all areas |
X100 |
|
Total number of days monitored |
· Frequency: Monthly.
1.2.3 PPE Stock-out Incidence Rate
· KPI
Definition: The number of PPE stock-out incidents over a given period.
· Target: Each organization
should set its target according to the strength of recommendation and gap
analysis.
· Calculation:
|
Stock out Incidence Rate = |
Number of Stock out Incidence |
X100 |
|
Total number of days in the monitoring period |
· Frequency: Quarterly.
1.2.4 PPE Training Completion Rate
· KPI Definition: The percentage of healthcare workers who have completed PPE training within a specified period.
· Target: Each organization should set its target according to the strength of recommendation and gap analysis.
· Calculation:
|
Training Completion Rate = |
Number of staff completing training |
X100 |
|
Total number of staff required to train |
· Frequency: Annually.
1.2.5 PPE Incident Reporting Rate
· KPI Definition: The number of incidents of PPE misuse or failure reported per 1,000 patient days.
· Target: Each organization should set its target according to the strength of recommendation and gap analysis.
· Calculation:
|
PPE Incident Reporting Rate = |
Number of PPE incidents reported |
X100 |
|
Total number of patient days |
· Frequency: Monthly.
These KPIs are designed to provide measurable and actionable insights into the effective management and use of PPE in infection prevention and control settings. Regular monitoring of these KPIs will help ensure adherence to protocols, sufficient PPE availability, and overall safety for healthcare workers and patients.
The tools mentioned for monitoring and evaluating the use of PPE in infection prevention and control are developed based on general best practices from established guidelines and frameworks.
1.3 Plan to Update this National Clinical Guideline
This guideline will be updated whenever there is new evidence.
- Annexes
Annex 1. How to remove gloves

Annex 2. The Glove Pyramid

Annex 3. Sequence for Putting and removing on PPE (Quoted from CDC)

- References
- PPE ref CDC Summary of Recommendations guidelines for isolation precautions preventing transmission of infectious agents in healthcare setting 2007 updated Nov 2023
- Occupational Safety and Health Administration. (2021). COVID-19 Healthcare Emergency Temporary Standard
- Centers for Disease Control and Prevention (CDC). (2020). Personal Protective Equipment (PPE) Burn Rate Calculator. Retrieved from https://www.cdc.gov
- Centre for Disease Control and Prevention. (2020). Personal protective equipment (PPE) Burn Rate Calculator and Resources. Retrieved from: https://www.cdc.gov
- European Centre for Disease Prevention and Control. (2020). Infection prevention and control for COVID-19 in healthcare settings. Retrieved from: https://www.ecdc.europa.eu
- Occupational Safety and Health Administration (OSHA). (2020). Personal protective equipment standards. Retrieved from https://www.osha.gov
- Public Health England. (2020). Guidance on the use of personal protective equipment (PPE) in hospitals during the COVID-19 outbreak. Retrieved from https://www.gov.uk
- World Health Organization. (2020). Rational use of personal protective equipment (PPE) for coronavirus disease (COVID-19) and considerations during severe shortages. Retrieved from https://www.who.int
- World Health Organization. (2020). Rational use of personal protective equipment (PPE) for coronavirus disease (COVID-19) and considerations during severe shortages. Retrieved from: https://www.who.int
- Infection Prevention and Control Canada. (2019). Infection Prevention and Control Guidelines and PPE Monitoring Tools. Retrieved from: https://ipac-canada.org
- World Health Organization. (2016). Guidelines on core components of infection prevention and control programs at the national and acute health care facility level. Retrieved from https://www.who.int