Hand Hygiene
| Site: | EHC | Egyptian Health Council |
| Course: | Infection Prevention and Control Guidelines |
| Book: | Hand Hygiene |
| Printed by: | Guest user |
| Date: | Monday, 23 December 2024, 9:06 PM |
Description
"last update: 28 Oct 2024"
- Acknowledgements
We would like to acknowledge the Infection Control Guidelines Committee for developing these guidelines.
▪️ Head of IPC Guidelines Committee
Prof Dr Ghada Ismail (Professor of Clinical Pathology (Clinical Microbiology), Faculty of Medicine, Ain Shams University, Secretary of Supreme IPC Committee, SCUH, Member of WHO Global Guidelines Groups (GDG) for Infection Prevention)
▪️ Secretary of IPC Guidelines Committee
Prof Dr Walaa Abd El-Latif (Professor of Medical Microbiology and Immunology, Faculty of Medicine Ain Shams University, IPC Consultant)
▪️ Members of the Committee
- Prof Dr Amal Sayed (Deputy Manager of Environmental Affair, Infection Control Director, Cairo University Hospitals)
- Prof Dr Amani El-Kholy (Clinical Pathology Department (Microbiology), Faculty of Medicine, Cairo University, Infection Control Consultant)
- Dr Gehan Mohamed Fahmy (Professor clinical microbiology ASUSH consultant infection control, Board member of IFIC EMERO region)
- Prof Dr Hebatallah Gamal Rashed (Clinical Pathology Department (Microbiology), Faculty of Medicine, Assuit University, Infection Control Consultant)
- Dr Iman Afifi (Consultant Clinical Pathology (Microbiology) and IPC, Ain Shams University, Director IPC units of Ain Shams internal medicine and Geriatric hospitals
- Prof Dr Maha El Touny (Department of internal medicine. Faculty of Medicine, Ain Shams University. Infection Control Consultant)
- Prof Dr Nagwa Khamis (Emeritus Consultant Clinical Pathology (Microbiology) and IPC, ASU Director IPC Department and CEO Consultant IPC, CCHE-57357)
- Prof Dr Nesrine Fathi Hanafi (Professor in Medical Microbiology and Immunology Faculty of Medicine Alexandria University, Head of Infection Prevention and Control, Alexandria University Hospitals)
- Dr. Reham Lotfy Abdel Aziz (Environmental Health Director, EEAA, Hazardous Waste Consultant, WMRA, Ministry of Environment)
- Prof Dr Sherin ElMasry (Professor of Clinical Pathology, Ain Shams University, Chief Director of IPC ASU, Health Care Quality & Patient Safety Consultant)
- Dr Shimaa El-Garf (Coordinator): Clinical Pathology Specialist, Coordinator of HAI Surveillance and Audit Electronic System for University Hospitals, RLEUH- SCUH
- List of Abbreviations
ABHR: Alcohol-based hand Rub
CDC: Centers for
Disease Control and Prevention
HCWs: Health-care
workers
IPC: Infection
prevention and control
WHO: World Health
Organization
- Glossary of Terms
- Alcohol based hand rub (ABHR): A gel, foam or liquid containing one or more types
of alcohol that is rubbed into the hands to inactivate microorganisms and/or
temporarily suppress their growth.
- Body Fluids:
Fluid produced by the body such as urine, faeces, vomit or diarrhea.
- Decontamination: A process, or combination of processes that removes or
destroys contamination on an item or surface to make it safe for handling,
re-use or disposal, by preventing infectious agents or other contaminants
reaching a susceptible site in sufficient quantities to initiate infection, or
other harmful response. Decontamination may include cleaning,
disinfection and/or sterilization.
- Hand Hygiene: The process of decontaminating your hands using either
alcohol-based hand rub or liquid soap and water.
- Invasive Medical Device: Any medical device that enters the body either
through a body opening or through a skin or mucous membrane breaking.
- Hand Hygiene in Healthcare Executive Summary
Guidelines on Hand Hygiene in Health Care provide health-care workers (HCWs), hospital administrators and health authorities with a thorough review of evidence on hand hygiene in health care and specific recommendations to improve practices and reduce transmission of pathogenic microorganisms to patients and HCWs. The present Guidelines are intended to be implemented in any situation in which health care is delivered either to a patient or to a specific group in a population. Therefore, this concept applies to all settings where health care is permanently or occasionally performed.
|
Serial |
Recommendations |
|
1 |
Indications for hand hygiene a. Wash hands with soap and water when: 1. Visibly dirty or visibly soiled with blood or other body fluids or after using the toilet (Strong recommendation) 2. If exposure to potential spore-forming pathogens is strongly suspected or proven, including outbreaks of Clostridium difficile (Strong recommendation) b. Use an alcohol-based handrub as the preferred means for hand antisepsis in all other clinical situations, if hands are not visibly soiled (Strong recommendation) c. Perform hand hygiene 1. before and after touching the patient (Strong recommendation) 2. before handling an invasive device for patient care, regardless of whether or not gloves are used (Strong recommendation) 3. after contact with body fluids or excretions, mucous membranes, non-intact skin, or wound dressings (Strong recommendation) 4. if moving from a contaminated body site to another body site during care of the same patient (Strong recommendation) 5. after contact with inanimate surfaces and objects (including medical equipment) in the immediate vicinity of the patient after removing sterile or non-sterile gloves (Strong recommendation) d. Before handling medication or preparing food perform hand hygiene using an alcohol-based handrub or wash hands with soap and water. (Strong recommendation) |
|
2 |
Hand hygiene technique a. Apply a palm-full of alcohol-based handrub and cover all surfaces of the hands. Rub hands until dry. (Strong recommendation) b. When washing hands with soap and water, wet hands with water and apply the amount of product necessary to cover all surfaces. Rinse hands with water and dry thoroughly with a single-use towel. Use clean, running water whenever possible. Make sure towels are not used multiple times or by multiple people. (Strong recommendation) |
|
3 |
Surgical hand preparation a. Remove rings, wrist-watch, and bracelets before beginning surgical hand preparation. (Good practice statement) Artificial nails are prohibited. (Strong recommendation) b. If hands are visibly soiled, wash hands with plain soap before surgical hand preparation. (Conditional recommendation) c. Remove debris from underneath fingernails using a nail cleaner, preferably under running water. (Good practice statement) d. Sinks should be designed to reduce the risk of splashes (Good practice statement) e. Brushes are not recommended for surgical hand preparation. (Conditional recommendation) f. Surgical hand antisepsis should be performed using either a suitable antimicrobial soap or suitable alcohol-based handrub, preferably with a product ensuring sustained activity, before donning sterile gloves. (Strong recommendation) g. When performing surgical hand antisepsis using an antimicrobial soap, scrub hands and forearms for the length of time recommended by the manufacturer, typically 2–5 minutes. Long scrub times (e.g. 10 minutes) are not necessary. (Strong recommendation) h. When using an alcohol-based surgical handrub product with sustained activity, follow the manufacturer’s instructions for application times. Apply the product to dry hands only and allow hands and forearms to dry thoroughly before donning sterile gloves. (Strong recommendation) |
|
4 |
Use of gloves a. The use of gloves does not replace the need for hand hygiene by either hand rubbing or handwashing. (Strong recommendation) |
|
5 |
Selection and handling of hand hygiene agents a. Provide HCWs with efficacious hand hygiene products that have low irritancy potential. (Strong recommendation) b. To maximize acceptance of hand hygiene products by HCWs, solicit their input regarding the skin tolerance feel, and fragrance of any products under consideration. (Strong recommendation) c. When selecting hand hygiene products: 1. determine any known interaction between products used to clean hands, skin care products, and the types of gloves used in the institution. (Good practice statement) 2. solicit information from manufacturers about the risk of product contamination. (Strong recommendation) 3. ensure that dispensers are accessible at the point of care. (Strong recommendation) 4. ensure that dispenser function adequately and reliably and deliver an appropriate volume of the product. (Good practice statement) 5. ensure that the dispenser system for alcohol-based handrubs is approved for flammable materials. (Conditional recommendation). 6. solicit and evaluate information from manufacturers regarding any effect that hand lotions, creams, or alcohol-based handrubs may have on the effects of antimicrobial soaps being used in the institution. (Strong recommendation) d. Do not add soap to a partially empty soap dispenser. If soap dispensers are reused, follow recommended procedures for cleansing. (Strong recommendation) |
|
6 |
Educational and motivational programs for health-care workers 1. In hand hygiene promotion programs for HCWs, focus specifically on factors currently found to have a significant influence on behaviour, and not solely on the type of hand hygiene products. The strategy should be multifaceted and multimodal and include education and senior executive support for implementation. (Strong recommendation) 2. Educate HCWs about the type of patient-care activities that can result in hand contamination and about the advantages and disadvantages of various methods used to clean their hands. (Good practice statement) 3. Monitor HCWs’ adherence to recommended hand hygiene practices and provide them with performance feedback. (Strong recommendation) 4. Encourage partnerships between patients, their families, and HCWs to promote hand hygiene in health care settings. (Good practice statement) |
- Introduction
Hands
are a primary source of infection transmission in all community, health care
settings and residential contexts, as well as in industrial settings like the
food sector. So, it is impossible to undervalue the significance of hand
cleanliness in the prevention of infection.
Even
though hand hygiene has received more attention in the medical literature,
there are still a lot of unanswered and unresolved inquiries and questions.
Hand
hygiene is the cornerstone of most of the infection
prevention and control (IPC) programs since it is recognized as the single
most significant way to stop the spread of infection.
Promoting
good hand hygiene is essential for both staff and patient safety. HCWs must be
knowledgeable about hand hygiene guidelines and continuously follow them for
patient safety and infection prevention and control strategies to be effective.
HCWs still
generally have poor compliance with hand hygiene regulations, despite that many
countries have established or adopted hand hygiene guidelines. For infection
preventionist in all healthcare settings, improving hand hygiene is still a
struggle and ongoing challenge.
Lack
of knowledge, increased demands with less time, dry and irritated hands, lack
of soap and paper towels, inaccessible sinks, lack of sinks, forgetfulness,
doubting the benefits of handwashing, absence of role models, lack of
administrative priority for hand hygiene, and absence of administrative
sanctions are some of the factors contributing to poor adherence and
compliance.
HCWs
use alcohol-based hand sanitizers for the past 20 years. Alcohol-based hand rub
(ABHR) should be used preferentially, and in all healthcare, facilities should
monitor and improve hand hygiene, according to the World
Health Organization's (WHO) 2009 hand hygiene guidelines.
The
current standard for regular hand hygiene in healthcare settings is to use
alcohol-based, waterless hand rubs, unless the hands are obviously dirty.
Facilities should supply an easily accessible alcohol-based hand sanitizer
product to HCWs, according to guidelines from the Centers for Disease Control
and Prevention (CDC) and WHO.
- Scope and Purpose
The guideline applies to all staff, patients,
patients’ relatives, and visitors. It emphasizes the importance of hand hygiene
in preventing the spread of infection and provides clear instructions on how to
perform it correctly. The goal is to help all staff, patients, patients’
relatives, and visitors implement best practices for infection control. In all
areas of the hospital whether medical or non-medical.
Effective hand hygiene removes harmful
microorganisms from the hands. It helps to reduce the risk of
cross-contamination between patients, equipment, and the environment. Hand
hygiene is the most important strategy for preventing the transmission of organisms.
Cleaning hands thoroughly between patient contact and after contact with bodily
fluids and patient zone is essential for preventing healthcare-associated
infections.
➡️Target
Audience
- Healthcare settings staff (physician, nurse, pharmacists, dentists, technicians,
housekeepers, auxiliary services staff, administrative, etc)
-
IPC practitioners
-
Policy- and decision-makers
-
Patients and families
- The community
- Methodology
A comprehensive search for guidelines was undertaken to identify the most relevant guidelines to consider for adaptation.
Inclusion/ exclusion criteria followed in the search and retrieval of guidelines to be adapted:
▪️ Selecting only evidence-based guidelines (guideline must include a report on systematic literature searches and explicit links between individual recommendations and their supporting evidence)
▪️ Selecting only national and/or international guidelines
▪️ Specific range of dates for publication (using Guidelines published or updated in 2013 and later)
▪️ Selecting peer reviewed publications only
▪️ Selecting guidelines written in English language
▪️ Excluding guidelines written by a single author, not on behalf of an organization to be valid and comprehensive, a guideline ideally requires multidisciplinary input.
▪️ Excluding guidelines published without references as the panel needs to know whether a thorough literature review was conducted and whether current evidence was used in the preparation of the recommendations.
The following characteristics of the retrieved guidelines were summarized in:
- Developing organisation/authors
- Date of publication, posting, and release
- Country/language of publication
- Date of posting and/or release
- Dates of the search used by the source guideline developers.
All retrieved Guidelines were screened and appraised using AGREE II instrument (www.agreetrust.org) by at least three members. The panel decided on a cut-off point or ranked the guidelines (any guideline scoring above 50% on the rigor dimension was retained). The committee decided to adapt from
1. WHO Guidelines on Hand Hygiene in Health Care 2009
2. Infection Prevention and Control (IPC) National Irish Clinical Guideline No. 30 May 2023 Vol 1
3. Guideline for Hand Hygiene in Health-Care Settings Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force 2002, updated March 2024
➡️Evidence assessment
According to WHO Handbook for Guidelines, we used the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach to assess the quality of a body of evidence, develop and report recommendations. GRADE methods are used by WHO because these represent internationally agreed standards for making transparent recommendations. Detailed GRADE information is available on the following sites:
▪️ GRADE working group: http://www.gradeworkingroup.org
▪️ GRADE online training modules: http://cebgrade.mcmaster.ca/
▪️ GRADE profile software: http://ims.cochrane.org/revman/gradepro
Table (1) Quality and Significance of the four levels of evidence in GRADE
|
Quality |
Definition |
Implications |
|
High |
The guideline development group is very confident that the true effect lies close to that of the estimate of the effect |
Further research is very unlikely to change confidence in the estimate effect |
|
Moderate |
The guideline development group is moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibly that it is substantially different |
Further research is likely to have an important impact on confidence in the estimate of the effect and may change the estimate |
|
Low |
Confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the true effect |
Further research is very likely to have an important on confidence in the estimate of effect and is unlikely to change the estimate |
|
Very low |
The group has very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect |
Any estimate of the effect is very uncertain |
|
Downgrade in presence of |
Upgrade in presence of |
|
Study limitations. 1- Serious limitations 2- Very serious limitations |
Dose- response gradient. +1 Evidence of a dose-response gradient |
|
Consistency 1- Important inconsistency |
Direction of plausible bias + All plausible confounders would have reduced the effect |
|
Directness 1- Some uncertainty 2- Major uncertainty |
Magnitude of the effect +1 Strong, no plausible Confounder, consistent and direct evidence |
|
Precision 1- Imprecise data |
+2 very strong, no major threats to validity and direct evidence |
|
Reporting bias 1- High probability of reporting bias |
|
➡️The strength of the recommendations
The strength of a recommendation communicates the importance of adherence to the recommendation.
▪️ Strong recommendations
With strong recommendations, the guideline communicates the message that the desirable effects of adherence to the recommendation outweigh the undesirable effects. This means that in most situations the recommendation can be adopted as policy.
▪️ Conditional recommendations
These are made when there is greater uncertainty about the four factors above or if local adaptation has to account for a greater variety in values and preferences, or when resource use makes the intervention suitable for some, but not for other locations. This means that there is a need for substantial debate and involvement of stakeholders before this recommendation can be adopted as policy.
When not to make recommendations?
When there is lack of evidence on the effectiveness of an intervention, it may be appropriate not to make a recommendation.
- Recommendations
|
Serial |
Recommendations |
|
1 |
Indications for hand hygiene a. Wash hands with soap and water when: 1. Visibly dirty or visibly soiled with blood or other body fluids or after using the toilet (Strong recommendation, High grade evidence) 2. If exposure to potential spore-forming pathogens is strongly suspected or proven, including outbreaks of Clostridium difficile (Strong recommendation, Moderate grade evidence) b. Use an alcohol-based handrub as the preferred means for hand antisepsis in all other clinical situations, if hands are not visibly soiled (Strong recommendation, High grade evidence) c. Perform hand hygiene 1. before and after touching the patient (Strong recommendation, Moderate grade evidence) 2. before handling an invasive device for patient care, regardless of whether or not gloves are used (Strong recommendation, Moderate grade evidence) 3. after contact with body fluids or excretions, mucous membranes, non-intact skin, or wound dressings (Strong recommendation, High grade evidence) 4. if moving from a contaminated body site to another body site during care of the same patient (Strong recommendation, Moderate grade evidence) 5. after contact with inanimate surfaces and objects (including medical equipment) in the immediate vicinity of the patient after removing sterile or non-sterile gloves (Strong recommendation, Moderate grade evidence) d. Before handling medication or preparing food perform hand hygiene using an alcohol-based handrub or wash hands with soap and water. (Strong recommendation, Moderate grade evidence) |
Remarks
When to Perform Hand Hygiene
The 5 moments of hand hygiene developed by the WHO to provide safe healthcare services for both people receiving and workers providing healthcare services from acquiring infectious microorganisms. (Annex 1).
Table (3): Methods for Hand Hygiene
Alcohol-Based Hand Rub/ Wash with soap and water |
Wash with soap and water |
Immediately before touching a patient |
When hands are visibly soiled |
Before performing an aseptic task (e.g., placing an indwelling device) or handling invasive medical devices |
After caring for a person with known or suspected infectious diarrhea |
Before moving from work on a soiled body site to a clean body site on the same patient |
After known or suspected exposure to spores (e.g. B. anthracis, C difficile outbreaks) |
After touching a patient or the patient’s immediate environment |
After contact with blood, body fluids or contaminated surfaces |
Immediately after glove removal |
- Before start and leaving work
- Before eating or handling food
- Before and after use of computer keyboards, tablets, mobiles or devices surrounding the patient.
- Before and after visiting the toilet
- Before wearing gloves not to contaminate them
- After removing gloves to decontaminate hands from splash while taking off gloves
- Handling laundry, waste or equipment
- Blowing/wiping/touching nose and mouth
|
Serial |
Recommendations |
|
2 |
Hand hygiene technique a. Apply a palm-full of alcohol-based handrub and cover all surfaces of the hands. Rub hands until dry. (Strong recommendation, Moderate grade evidence) b. When washing hands with soap and water, wet hands with water and apply the amount of product necessary to cover all surfaces. Rinse hands with water and dry thoroughly with a single-use towel. Use clean, running water whenever possible. Make sure towels are not used multiple times or by multiple people. (Strong recommendation, Moderate grade evidence) |
|
Serial |
Recommendations |
|
3 |
Surgical hand preparation a. Remove rings, wrist-watch, and bracelets before beginning surgical hand preparation. (Good practice statement) Artificial nails are prohibited. (Strong recommendation, Moderate grade evidence) b. If hands are visibly soiled, wash hands with plain soap before surgical hand preparation. (Conditional recommendation, Moderate grade evidence) c. Remove debris from underneath fingernails using a nail cleaner, preferably under running water. (Good practice statement) d. Sinks should be designed to reduce the risk of splashes (Good practice statement) e. Brushes are not recommended for surgical hand preparation. (Conditional recommendation, Moderate grade evidence) f. Surgical hand antisepsis should be performed using either a suitable antimicrobial soap or suitable alcohol-based handrub, preferably with a product ensuring sustained activity, before donning sterile gloves. (Strong recommendation, Moderate grade evidence) g. When performing surgical hand antisepsis using an antimicrobial soap, scrub hands and forearms for the length of time recommended by the manufacturer, typically 2–5 minutes. Long scrub times (e.g. 10 minutes) are not necessary. (Strong recommendation, Moderate grade evidence) h.When using an alcohol-based surgical handrub product with sustained activity, follow the manufacturer’s instructions for application times. Apply the product to dry hands only and allow hands and forearms to dry thoroughly before donning sterile gloves. (Strong recommendation, Moderate grade evidence) |
Hand Hygiene for Surgery
- Surgical hand antisepsis
▪️ Performing surgical hand antisepsis using either an antimicrobial soap or an alcohol-based hand sanitizer with persistent activity is recommended before donning sterile gloves when performing surgical procedures.
▪️ When using an alcohol-based surgical hand-scrub product with persistent activity, follow the manufacturer’s instructions.
▪️ Before applying the alcohol solution, prewash hands and forearms with a non-antimicrobial soap and dry hands and forearms completely
▪️ After application of the alcohol-based product as recommended, allow hands and forearms to dry thoroughly before donning sterile gloves.
|
Serial |
Recommendations |
|
4 |
Use of gloves a. The use of gloves does not replace the need for hand hygiene by either hand rubbing or handwashing. (Strong recommendation, Moderate grade evidence) |
|
5 |
Selection and handling of hand hygiene agents a. Provide HCWs with efficacious hand hygiene products that have low irritancy potential. (Strong recommendation, Moderate grade evidence) b.To maximize acceptance of hand hygiene products by HCWs, solicit their input regarding the skin tolerance feel, and fragrance of any products under consideration. (Strong recommendation, Moderate grade evidence) c. When selecting hand hygiene products: 1. determine any known interaction between products used to clean hands, skin care products, and the types of gloves used in the institution. (Good practice statement) 2. solicit information from manufacturers about the risk of product contamination. (Strong recommendation, Moderate grade evidence) 3. ensure that dispensers are accessible at the point of care. (Strong recommendation, Moderate grade evidence) 4. ensure that dispenser function adequately and reliably and deliver an appropriate volume of the product. (Good practice statement) 5. ensure that the dispenser system for alcohol-based handrubs is approved for flammable materials. (Conditional recommendation, low grade evidence) 6. solicit and evaluate information from manufacturers regarding any effect that hand lotions, creams, or alcohol-based handrubs may have on the effects of antimicrobial soaps being used in the institution. (Strong recommendation, Moderate grade evidence) d. Do not add soap to a partially empty soap dispenser. If soap dispensers are reused, follow recommended procedures for cleansing. (Strong recommendation, Moderate grade evidence) |
|
6 |
Educational and motivational programs for health-care workers 1. In hand hygiene promotion programs for HCWs, focus specifically on factors currently found to have a significant influence on behaviour, and not solely on the type of hand hygiene products. The strategy should be multifaceted and multimodal and include education and senior executive support for implementation. (Strong recommendation, High grade evidence) 2. Educate HCWs about the type of patient-care activities that can result in hand contamination and about the advantages and disadvantages of various methods used to clean their hands. (Good practice statement) 3. Monitor HCWs’ adherence to recommended hand hygiene practices and provide them with performance feedback. (Strong recommendation, High grade evidence) 4. Encourage partnerships between patients, their families, and HCWs to promote hand hygiene in health care settings. (Good practice statement) |
1.1.1.1 Washing hands with soap and water is required if hands are visibly soiled while either product can be used if hands are visibly clean.
1.1.1.2 Minimize physical contact with patient surroundings.
1.1.1.3 Whenever there is shortage in hand soap, drying paper or hand rub this must be brought up to the responsible in the department and IPC specialist
1.1.1.4 Wearing jewelry, watches, rings, artificial fingernails, or nail polish by healthcare workers can compromise performance of optimal hand hygiene.
1.1.1.5 Intact skin is a natural defense against infection. Cuts, abrasions, fingernail or hand skin disease can reduce the effectiveness of hand hygiene practices and can be sources of infectious microorganisms. So, it is advised to cover those areas with waterproof dressings.
1.1.1.6 Fingernails should therefore be kept short (the length of the finger pad) and clean, and artificial fingernails should not be worn. Nail polish/varnish should not be used; particularly as chipped nail polish may support the growth of microorganisms on the fingernail.
1.1.1.7 It is better not use crude 70% alcohol to perform hand hygiene practices as it does not achieve the proper contact time required during hand rub and its repeated use leads to excessive dryness and inflammation e.g. contact dermatitis can take place leading to diseased skin.
1.1.1.8 Appropriate use of hand lotion or moisturizers added to hand hygiene preparations is an important factor in maintaining skin integrity, encouraging adherence to hand hygiene practices and assuring the health and safety of healthcare workers.
1.1.1.9 Healthcare workers should be educated about the risk of irritant contact dermatitis and in case of allergy from a hand hygiene disinfectant or soap, and in case it is encountered it should be discussed with the manager and IPC specialist to find a proper substitute.
1.1.1.10 Extending awareness on hand hygiene practices to general population individuals who attend healthcare settings: visitors or users of services to receive medical and health services is also important in reducing significantly rates of healthcare associated infection, spread of communicable diseases and spread of multidrug resistant bacteria whether in the medical settings or in the general community.
1.1.1.11 It is important to ensure effectiveness by choosing an appropriate product (as per standards noted above) using a sufficient amount of product which allows complete coverage of the hands and allowing the hands to remain wet for the recommended amount of time, as per manufacturer instructions Alcohol-based hand rub should be readily available in work areas and near patients to increase accessibility unless the ease of access to alcohol poses a specific risk to individual patients. The following alcohol-based hand rub features are important in influencing acceptability:
▪️ Appealing fragrance is not mandatory while it is necessary to use emollient agents to prevent skin drying and irritant skin reactions, but not leave a sticky residue on hands. All hand hygiene products should be chemically compatible. It is advisable that hand hygiene and hand care products are from a range made by a single manufacturer as this can reduce risk of incompatibility between the products.
▪️ Other issues as cost issues, availability, convenience and functioning of dispenser and ability to prevent contamination and crusting of material at the dispenser tip. Practical information plain soap and water hand washing refers to the appropriate use of a non-antimicrobial soap and water on the surface of the hands.
▪️ Plain soaps act by mechanical removal of microorganisms and have no antimicrobial activity. They are suitable for performing hand hygiene and are required for cleansing of visibly soiled hands. They are also used for mechanical removal of certain organisms such as C. difficile and norovirus. Liquid soap dispensers are generally preferred to bar soap in healthcare settings. Antimicrobial soaps are sometimes used to decontaminate hands. However, when alcohol-based hand rub is available in the healthcare facility for hand hygiene, the use of antimicrobial soap is not recommended. Alcohol-based hand rubs are also suitable for use in resource limited or remote areas with lack of accessibility to sinks or other facilities for hand hygiene.
1.2 Indicators for Monitoring
1.2.1 Indicators for Monitoring Hand Hygiene in Hospitals
To ensure the effectiveness of hand hygiene practices in hospitals and reduce the risk of healthcare-associated infections (HAIs), specific indicators should be monitored regularly. These indicators provide measurable data to assess compliance, identify areas for improvement, and guide interventions. Here are some key indicators that can be included in hospital policy for monitoring hand hygiene :
1.2.1.1 Hand Hygiene Compliance Rate (Essential)
▪️ Definition: The percentage of observed hand hygiene opportunities where healthcare workers correctly perform hand hygiene according to established guidelines. Can be expressed also per HCW category and per location.
▪️ Calculation:
(Number of hand hygiene actions performed correctly / Total number of observed hand hygiene opportunities) × 100
- Target: Each organization should set its target according to the strength of recommendation and gap analysis.
- Importance: This is a direct measure of how well healthcare workers adhere to hand hygiene protocols, which is critical in preventing HAIs.
*In auxiliary services area that are not in direct contact with patients, we measure compliance rate by number of correct actions/ numbers of required actions.
1.2.1.2 Alcohol-Based Hand Rub (ABHR) Consumption (Optional)
- Definition: The volume of ABHR used per 1,000 patient-days.
- Calculation:
Total volume of ABHR consumed (in liters) / Total patient-days × 1000
- Target: Each organization should set its target according to the strength of recommendation and gap analysis.
- Importance: Monitoring ABHR consumption provides an indirect measure of hand hygiene activity, especially in high-risk areas.
1.2.1.3 Hand Hygiene Infrastructure Availability (For gap analysis)
- Definition: The percentage of patient care areas that have adequate hand hygiene facilities (e.g., sinks, ABHR dispensers) available and accessible.
- Calculation:
(Number of patient care areas with adequate facilities / Total number of patient care areas) × 100
- Target: Each organization should set its target according to the strength of recommendation and gap analysis.
- Importance: Adequate infrastructure is essential for enabling and sustaining high compliance with hand hygiene practices.
1.2.1.4 Hand Hygiene Knowledge and Perception (Orientation and training needs)
- Definition: The percentage of healthcare workers who demonstrate adequate knowledge of hand hygiene guidelines and perceive it as an essential practice.
- Calculation:
Based on survey data (Number of correct responses or positive perceptions / Total number of survey respondents) × 100
- Target: Each organization should set its target according to the strength of recommendation and gap analysis.
- Importance: Knowledge and perception influence behaviour; improving these aspects can enhance compliance.
1.2.1.5 Patient and Visitor Hand Hygiene Promotion (optional/ annual or according to healthcare settings policy)
- Definition: The extent of efforts made to educate patients and visitors about hand hygiene practices, including the availability of ABHR dispensers in public areas.
- Calculation: Based on observational data or surveys
(Number of educational sessions/materials provided / Total patient/visitor population) × 100
- Target: Each organization should set its target according to the strength of recommendation and gap analysis.
- Importance: Engaging patients and visitors in hand hygiene can help reduce the transmission of infections within the hospital.
### Conclusion
Incorporating these indicators into hospital guidelines for monitoring hand hygiene ensures a comprehensive approach to infection prevention. Regular monitoring, combined with effective feedback mechanisms, fosters a culture of continuous improvement in hand hygiene practices, ultimately leading to better patient outcomes and reduced HAIs.
These indicators align with recommendations from the WHO and other healthcare bodies, emphasizing the importance of both direct and indirect measures of hand hygiene performance.
1.3 Plan to Update this National Clinical Guideline
This guideline will be updated whenever there is new evidence.
- Annexes
 Figure (1): 5 moments for hand hygiene
Figure (1): 5 moments for hand hygiene
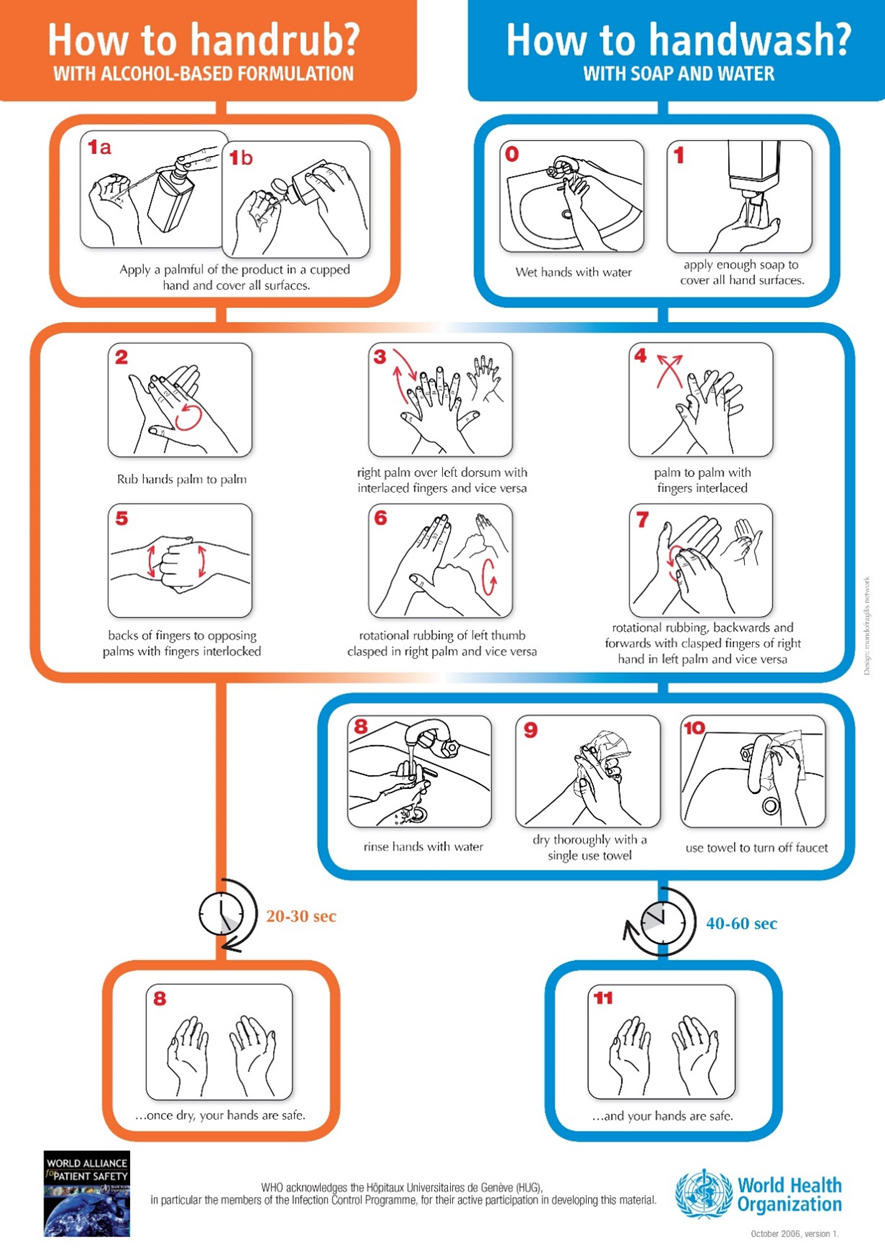
Figure (2): Hand rub/ Hand wash
WHO Hand Hygiene Observation Form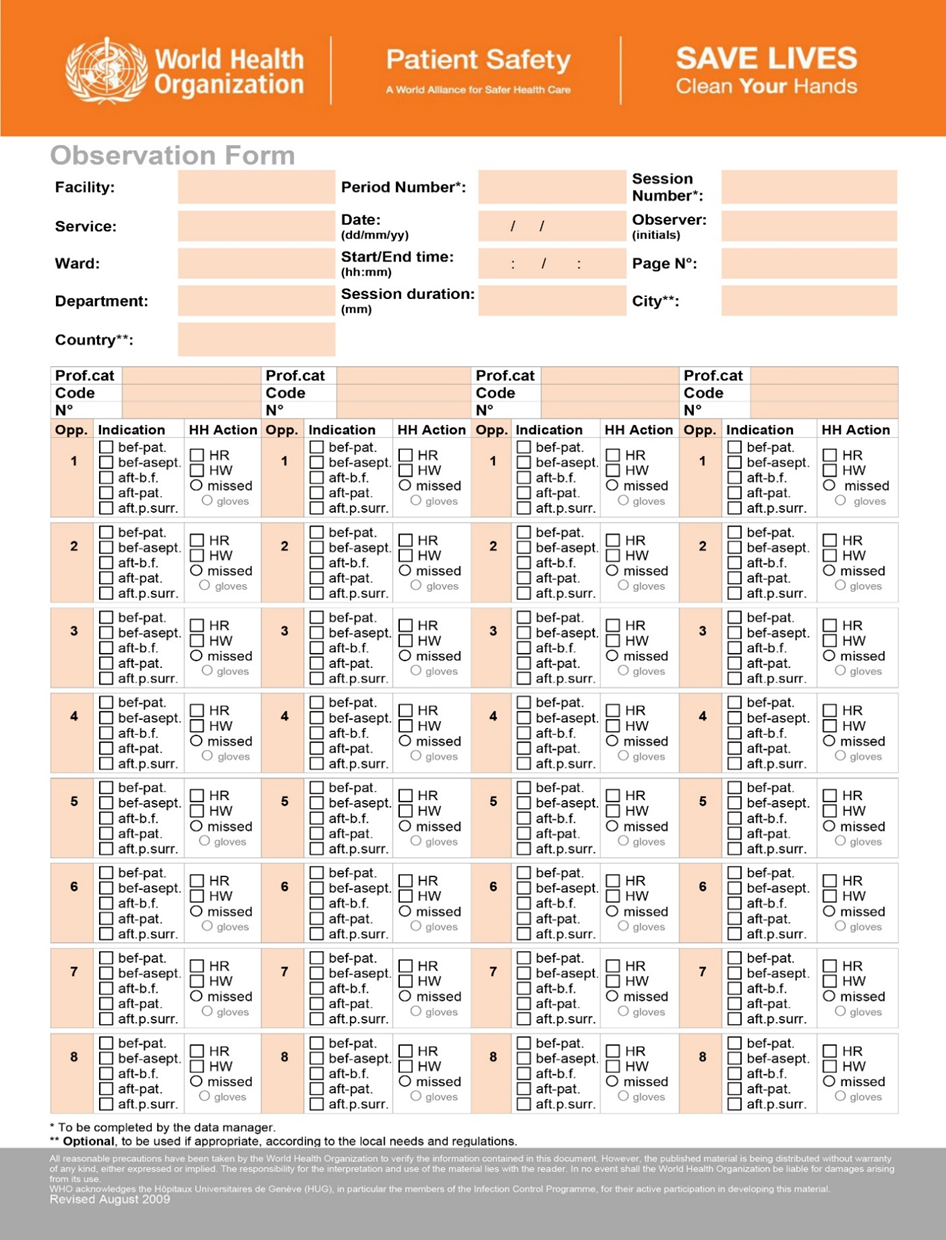

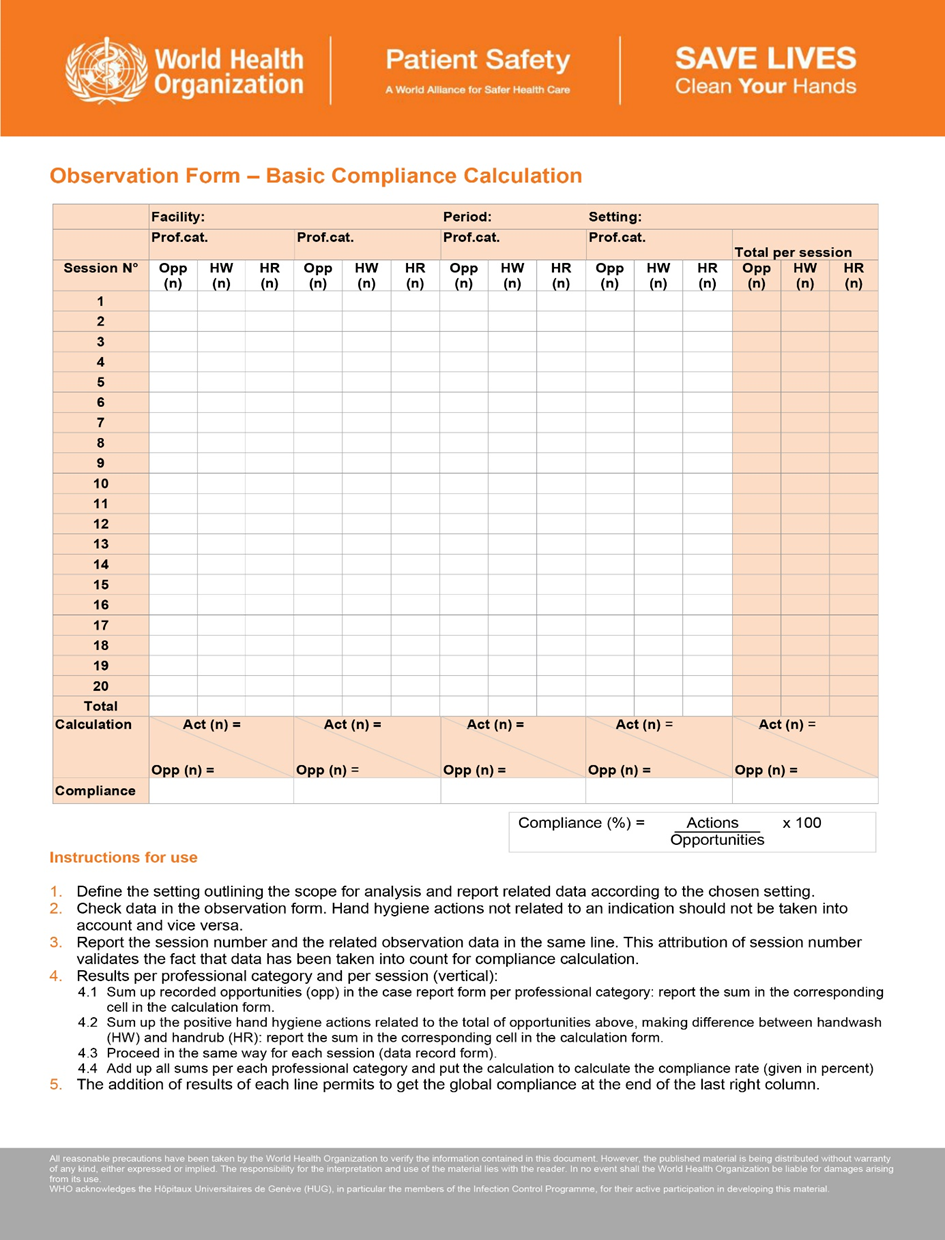
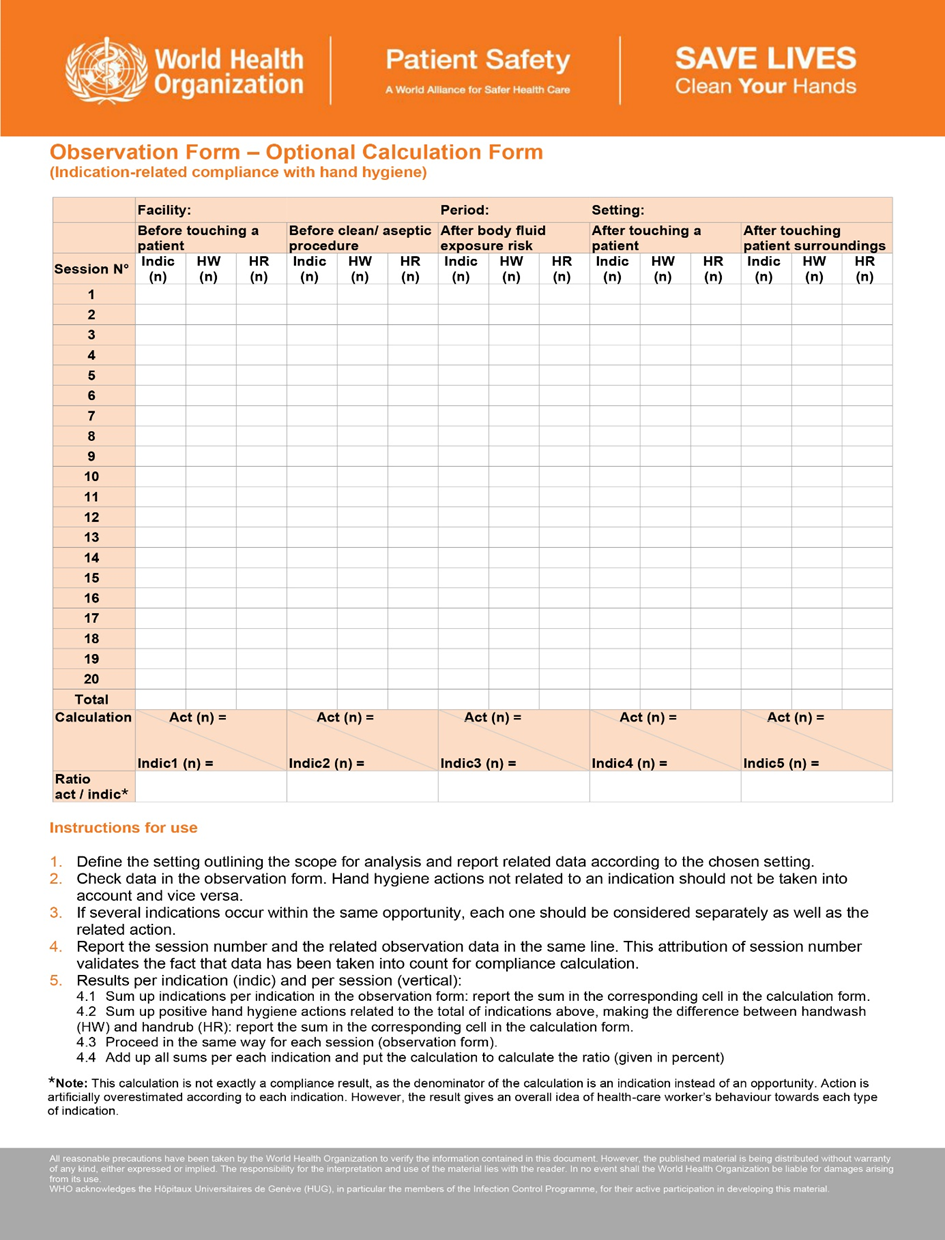
- Reference
1.WHO Guidelines on Hand Hygiene in Health Care 2009
2. Infection Prevention and Control (IPC) National Irish Clinical Guideline No. 30 May 2023 Vol 1
3. Guideline for Hand Hygiene in Health-Care Settings Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA
4. Hand Hygiene Task Force 2002,updated March 2024
5. NHS England. National infection prevention and control manual (NIPCM) for England. [Online].; 2024. Available from: https://www.england.nhs.uk/national-infection-prevention-and-control-manual-nipcm-for-england/.
6. WHO Regional Office for Europe. Infection prevention and control - guidance to action tools. Copenhagen; 2021. Report No.: ISBN: 978-92-890-5543-7.
7. Saskatchewan college of pharmacy professionals. Hand Hygiene Guidelines. 2024 March 13.
8. Department of Health. NCEC National Clinical Guideline No. 30 Infection Prevention Dublin: The Department of Health; 2023.
9. ohiniva AL, Bassim H, Hafez S, Kamel E, Ahmed E, Saeed T, et al. Determinants of hand hygiene compliance in Egypt: building blocks for a communication strategy. Eastern Mediterranean Health Journal. 2015; 21(9): p. 665-670.
10. Toney-Butler TJ, Gasner A, Carver N. Hand Hygiene Treasure Island (FL): StatPearls Publishing LLC; 2023.
11. Egyptian Ministry of Health. Egyptian Patient Safety Standards for Hospitals. 2nd ed.; 2013.