Ewing Sarcoma
| Site: | EHC | Egyptian Health Council |
| Course: | Pediatric Oncology Guidelines |
| Book: | Ewing Sarcoma |
| Printed by: | Guest user |
| Date: | Monday, 23 December 2024, 10:26 PM |
Description
"last update: 14 Oct 2024"
- Acknowledgment
We would like to acknowledge the Guidelines Development Group (GDG) of Paediatric Oncology for adapting this Guideline.
• Chair of the Committee:
Prof Alaa El-Haddad (Professor of Paediatric Oncology and Former Dean of the National Cancer Institute - Cairo University, Head of the Paediatric Oncology Department and the Bone Marrow Transplant Unit at the Children’s Cancer Hospital Cairo, Egypt).
• The Scientific Committee Members:
Prof Iman Sidhom (Professor and Head of Department of Paediatric Oncology - National Cancer Institute - Cairo University).
Prof Emad Ebied (Deputy Director, National Cancer Institute and Professor of Paediatric Oncology - National Cancer Institute - Cairo University).
Prof Mahmoud Hammad (Committee Rapporteur and Professor of Paediatric Oncology - National Cancer Institute - Cairo University - and Director of the Oncology and Nuclear Medicine Centre at Nasser Institute Hospital).
Prof Youssef Madney (Professor of Paediatric Oncology - National Cancer Institute - Cairo University - and Consultant of Paediatric Oncology at Dar Al Salam Cancer Hospital Harmal).
Dr Ahmed Mustafa (Lecturer of Paediatric Oncology - National Cancer Institute - Cairo University - and Assistant Head of the Specialized Medical Centres Secretariat for Oncology Affairs).
Dr Zaki Ahmed Zaki (Consultant and Director, Haematology Unit at Sheikh Zayed Specialized Hospital).
Dr Amal Ahmed Zein (Paediatric Oncology Consultant at Al-Sahel Teaching Hospital- and at Oncology Centre of Nasser Institute Hospital).
Prof Shady Fadel (Assistant Professor, Faculty of Medicine, Alexandria University - Director and Head, Paediatric Oncology Department, Borg El Arab Hospital).
Dr Mahmoud Motaz (Lecturer, South Egypt Oncology Institute, Assiut University, Medical Director and Head of the Paediatric Oncology Department at Shifa Al Orman Oncology Centre).
Dr Ebtehal Mahmoud Ali (Assistant lecturer of Paediatric Oncology at National Cancer Institute – Cairo University).
Dr Mai Mamdouh (Clinical Pharmacist, Paediatric Oncology, Dar El Salam Cancer Hospital Harmal).
- Abbreviations
AYA (Adolescent and Young Adult)
BM (bone marrow)
COG (Children’s Oncology Group)
CT (computed tomography)
FDG (fluorodeoxyglucose F18)
GCSF (granulocyte colony stimulating factor)
IHC (immunohistochemistry)
MRI (magnetic resonance imaging)
NCCN (National Comprehensive Cancer Network)
PET (positron emission tomography)
RT (radiation therapy)
- Glossary
Negative surgical margins: Minimal distance between the tumour and surgical margins is more than 2 mm. (1)
Positive surgical margins: Minimal distance between the tumour and surgical margins is 2 mm or less. (1)
- Executive Summary
This guidance provides a data-supported approach to the diagnosis, treatment, and follow up of paediatric patients diagnosed with Ewing sarcoma.
|
Recommendations
|
Strength Of recommendation |
|
1-Work up for newly diagnosed Ewing Sarcoma
|
|
|
Image guided biopsy with IHC is recommended.
|
Strong recommendation |
|
Molecular studies are recommended as needed guided by expert opinion.
|
Conditional recommendation |
|
Contrast enhanced MRI of the primary site is recommended.
|
Strong recommendation |
|
We recommend PET/CT if available or CT chest and bone scan if PET/CT is unavailable.
|
Strong recommendation |
|
Bone marrow biopsy is recommended if PET/CT is unavailable or positive uptake of bone marrow in PET/CT. |
Strong recommendation |
|
2- First line therapy for non-metastatic primary tumour (neoadjuvant/adjuvant)
|
|
|
Multiagent chemotherapy for at least 9 weeks prior to local therapy is recommended (interval compressed chemotherapy).
All patients are recommended to continue adjuvant chemotherapy after local control till 28 weeks. |
Strong recommendation
Strong recommendation |
|
Preferred Regimen VDC/IE (Vincristine, doxorubicin and cyclophosphamide) alternating with (Ifosfamide and etoposide) every 2 weeks with GCSF for a total of 14 cycles.
|
Strong recommendation |
|
Restage after neoadjuvant therapy before local control CT chest and contrast enhanced MRI of primary site are recommended.
|
Strong recommendation |
|
Local Control Therapy for stable/improved disease following neoadjuvant therapy
|
|
|
We recommend wide surgical excision and adjuvant chemotherapy. Radiotherapy is recommended if positive surgical margins. |
Strong recommendation |
|
Definitive radiotherapy and adjuvant chemotherapy are recommended for irresectable tumours.
|
Strong recommendation |
|
3- First line therapy for metastatic disease at initial presentation
|
|
|
Multiagent chemotherapy for at least 9 weeks prior to local therapy is recommended (interval compressed chemotherapy).
Preferred Regimen VDC/IE (Vincristine, doxorubicin and cyclophosphamide) alternating with (Ifosfamide and etoposide) every 2 weeks with GCSF for a total of 14 cycles.
All patients are recommended to continue adjuvant chemotherapy after local control till 28 weeks.
|
Strong recommendation
|
|
Local control for metastatic disease
|
|
|
We recommend wide surgical excision and adjuvant chemotherapy. Radiotherapy is recommended if positive surgical margins.
|
Strong recommendation |
|
Definitive radiotherapy and adjuvant chemotherapy are recommended for irresectable tumours.
|
Strong recommendation |
|
Management of metastases
|
|
|
For lung only metastases with partial response to neoadjuvant treatment, resection and whole lung irradiation are recommended.
|
Strong recommendation |
|
For lung only metastases with complete response to neoadjuvant treatment, whole lung irradiation is recommended.
|
Strong recommendation |
|
For bone metastases it is recommended to give radiotherapy to metastatic sites.
|
Strong recommendation |
|
4- Radiotherapy |
|
|
Timing of RT For patients receiving radiation therapy only it is recommended to be delivered at the beginning of week 13 concurrently with chemotherapy.
|
Strong recommendation |
|
If post-operative radiotherapy is recommended, consider starting at week 15 concurrently with chemotherapy starting on day 1 of the cycle as soon as possible after surgery.
|
Strong recommendation |
|
Patients with recent cord compression are recommended to start emergency concurrent radiotherapy and chemotherapy starting from day 1 first cycle.
|
Strong recommendation |
|
Concurrent chemotherapeutic agents Ifosfamide, etoposide, cyclophosphamide and vincristine should be given with radiotherapy. It is recommended to withhold doxorubicin with radiotherapy and re-institute after completion of radiation. |
Strong recommendation |
|
5- Treatment of recurrent/relapsed Ewing Sarcoma
|
|
|
Chemotherapy Recommended chemotherapy combination · Irinotecan and temozolomide in 21-day interval cycles, Or · Ifosfamide, carboplatin and etoposide (if > 6 months).
|
Strong recommendation |
|
Surgery Surgical resection of both local and metastatic sites (especially pulmonary) if feasible is recommended.
|
Strong recommendation |
|
Radiotherapy Radiation is recommended either definitive or postoperative.
|
Strong recommendation |
|
6- Surveillance – Follow up - for Ewing Sarcoma patients
|
|
|
X-ray of the primary site is recommended every 4 months for the first 2 years and as clinically warranted.
|
Strong recommendation |
|
CT chest every 4 months is the recommended chest imaging in the first 2 years. Chest X-ray is recommended for chest imaging in later years.
|
Strong recommendation |
|
It is recommended to increase intervals of imaging of primary site and chest after 24 months and annually after 5 years (indefinitely).
|
Strong recommendation |
- Introduction
Ewing sarcoma is the second most common primary bone tumour in paediatric population, accounting for about 1% of all childhood cancers. This malignancy typically originates in the bones or the surrounding soft tissues and most frequently affects AYAs, with a common age range at diagnosis between 10 and 20 years. The incidence of Ewing sarcoma is notably higher among individuals of European and North African/Middle Eastern ancestry, making it a significant concern in these populations. Despite its rarity, Ewing sarcoma is known for its aggressive nature and the challenges it presents in treatment and management. (2)(3)
- Scope of the Guideline
These guidelines are developed to improve the quality of care for Ewing Sarcoma cancer patients Via providing a uniform standard of care across the country to help in early diagnosis and treatment for Ewing Sarcoma, with improved clinical outcomes. These guidelines cover primary diagnosis, treatment and follow-up of Ewing Sarcoma patients.
Target audience
Clinicians who are involved in the care and treatment of patients with Ewing Sarcoma, including paediatric oncologists, radiation oncologists, surgeons, radiologists, pathologists, and palliative care specialists.
- Methodology
A comprehensive search for guidelines was undertaken to identify the most
relevant guidelines to consider for adaptation.
inclusion/exclusion criteria followed in the search and retrieval of
guidelines to be adapted:
- Selecting only evidence-based guidelines (guideline must include a
report on systematic literature searches and explicit links between
individual recommendations and their supporting evidence).
- Selecting only national and/or international guidelines.
- Specific range of dates for publication (using Guidelines published or
updated 2015 and later).
- Selecting peer reviewed publications only.
- Selecting guidelines written in English language.
- Excluding guidelines written by a single author not on behalf of an
organization in order to be valid and comprehensive, a guideline
ideally requires multidisciplinary input.
- Excluding guidelines published without references as the panel needs
to know whether a thorough literature review was conducted and
whether current evidence was used in the preparation of the
recommendations.
All retrieved Guidelines were screened and appraised using AGREE II
instrument (www.agreetrust.org) by at least two members. the panel decided
a cut-off points or rank the guidelines (any guideline scoring above 50% on
the rigour dimension was retained)
The NCCN guidelines are the main source used while formulating the national guidelines for Ewing Sarcoma.
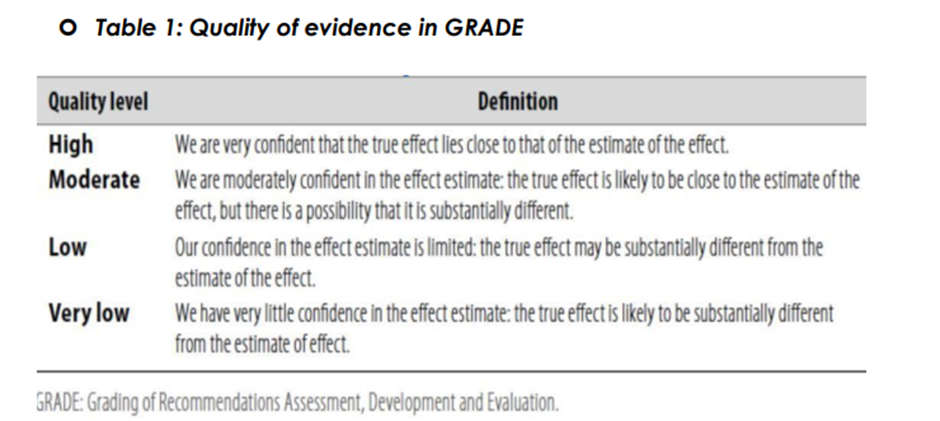
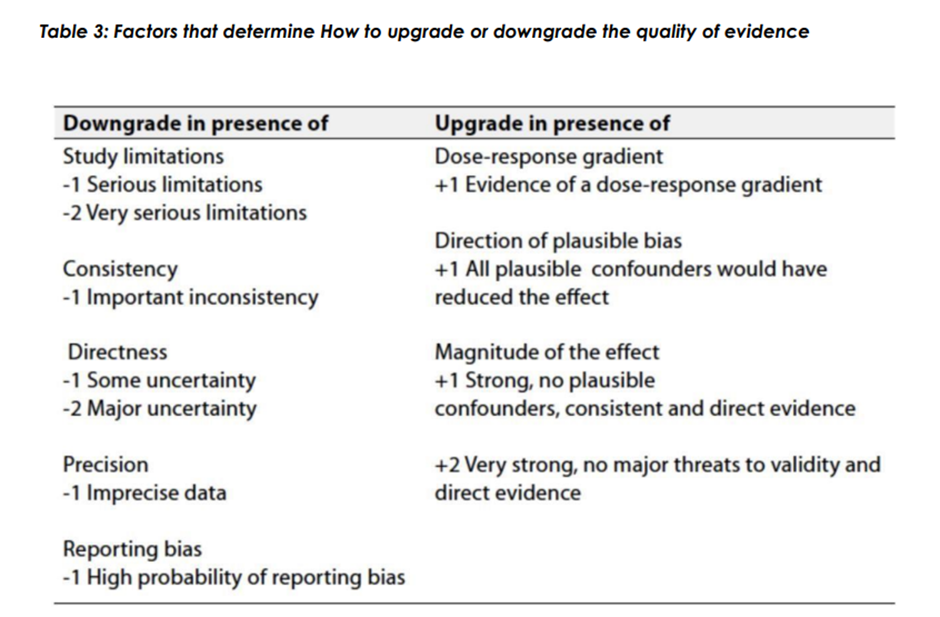
Evidence assessment
According to WHO handbook for Guidelines we used the GRADE (Grading
of Recommendations, Assessment, Development and Evaluation) approach
to assess the quality of a body of evidence, develop and report
recommendations. GRADE methods are used by WHO because these
represent internationally agreed standards for making transparent
recommendations. Detailed information on GRADE is available through the
on the following sites:
. GRADE working group: http://www.gradeworkingroup.org
. GRADE online training modules: http://cebgrade.mcmaster.ca/
. GRADE profile software: http://ims.cochrane.org/revman/gradepro
Table 1: Quality of evidence in GRADE
The strength of the recommendation
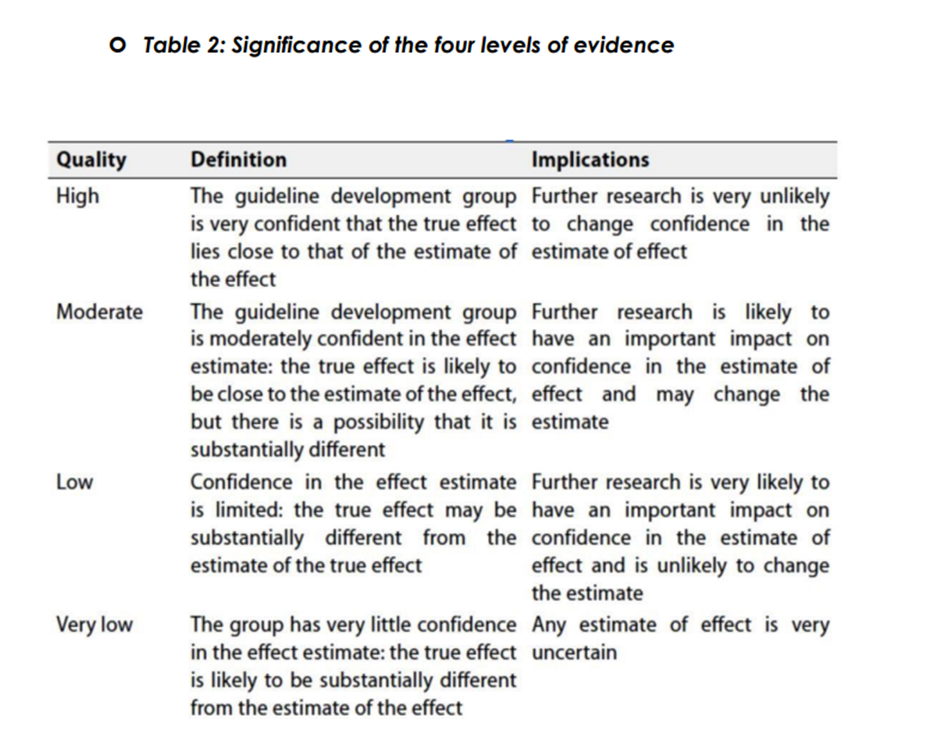
The strength of a recommendation communicates the importance of adherence to the recommendation:
Strong recommendations
With strong recommendations, the guideline communicates the message that
the desirable effects of adherence to the recommendation outweigh the
undesirable effects. This means that in most situations the recommendation
can be adopted as policy.
Conditional recommendations
These are made when there is greater uncertainty about the four factors
above or if local adaptation must account for a greater variety in values and
preferences, or when resource use makes the intervention suitable for some,
but not for other locations. This means that there is a need for substantial
debate and involvement of stakeholders before this recommendation can be
adopted as policy.
When not to make recommendations.
When there is lack of evidence on the effectiveness of an intervention, it may
be appropriate not to make a recommendation.
- Recommendations
1-Work up for newly diagnosed Ewing Sarcoma
Image guided biopsy with IHC is recommended.
strong recommendation, high quality level of evidence (systematic review and meta-analysis, comparative trial) (4)(5)
Molecular studies are recommended as needed guided by expert opinion.
Conditional recommendation, low quality level of evidence (retrospective analysis) (6)
Contrast enhanced MRI of the primary site is recommended.
strong recommendation, high quality level of evidence (comparative trial) (5)
We recommend PET/CT if available or CT chest and bone scan if PET/CT is unavailable.
strong recommendation, high quality level of evidence (systematic review and
meta-analysis) (4)
Bone marrow biopsy is recommended if PET/CT is unavailable or positive uptake of bone marrow in PET/CT.
strong recommendation, high quality evidence (systematic review) (7)
2- First line therapy for non-metastatic primary tumour
(neoadjuvant/adjuvant)
Multiagent chemotherapy for at least 9 weeks prior to local therapy is recommended (interval compressed chemotherapy).
strong recommendation, high quality level of evidence (randomised trials) (8-13)
All patients are recommended to continue adjuvant chemotherapy after local control till 28 weeks.
strong recommendation, high quality level of evidence (randomised trials) (8-13)
Preferred regimen
VDC/IE (Vincristine, doxorubicin and cyclophosphamide) alternating with (ifosfamide and etoposide) every 2 weeks with GCSF for a total of 14 cycles.
strong recommendation, high quality level of evidence (randomised trial) (13)
Restage after neoadjuvant therapy before local control
CT chest and contrast enhanced MRI of primary site are recommended.
strong recommendation, high quality level of evidence (COG report, randomised trial) (14)(15)
Local Control Therapy for stable/improved disease following neoadjuvant therapy
We recommend wide surgical excision and adjuvant chemotherapy. Radiotherapy is recommended if positive surgical margins.
strong recommendation, high quality level of evidence (retrospective analysis, COG report, prospective study) (16)(17)(18)
strong recommendation, high quality level of evidence (retrospective analysis, COG report, prospective study) (16)(17)(18)
3- First line therapy for metastatic disease at initial presentation
Multiagent chemotherapy for at least 9 weeks prior to local therapy is recommended (interval compressed chemotherapy).
Preferred Regimen
VDC/IE (Vincristine, doxorubicin and cyclophosphamide) alternating with (ifosfamide and etoposide) every 2 weeks with GCSF for a total of 14 cycles.
All patients are recommended to continue adjuvant chemotherapy after local control till 28 weeks.
strong recommendation, high quality level of evidence (randomised trial) (8-13)
Local control for metastatic disease
We recommend wide surgical excision and adjuvant chemotherapy. Radiotherapy is recommended if positive surgical margins.
strong recommendation, high quality level of evidence (retrospective analysis of clinical trial, retrospective analysis of clinical trial) (19)(20)
Definitive radiotherapy and adjuvant chemotherapy are recommended for irresectable tumours.
strong recommendation, high quality level of evidence (retrospective analysis of clinical trial, retrospective analysis of clinical trial) (19)(20)
Management of metastases
For lung only metastases with partial response to neoadjuvant treatment, resection and whole lung irradiation are recommended.
Strong recommendation, high quality level of evidence (retrospective analysis, Prospective multicentre trial) (21)(22)
For lung only metastases with complete response to neoadjuvant treatment, whole lung irradiation is recommended.
Strong recommendation, high quality level of evidence (retrospective analysis, Prospective multicentre trial) (21)(22)
For bone metastases it is recommended to give radiotherapy to metastatic sites.
(retrospective analysis) (23)
4- Radiotherapy
Timing of RT
For patients receiving radiation therapy only it is recommended to be delivered at the beginning of week 13 concurrently with chemotherapy.
strong recommendation, high quality level of evidence (systematic review, Prospective trial) (24)(25)
If post-operative radiotherapy is recommended, consider starting at week 15 concurrently with chemotherapy starting on day 1 of the cycle as soon as possible after surgery.
strong recommendation, high quality level of evidence (systematic review, Prospective trial) (24)(25)
Patients with recent cord compression are recommended to start emergency concurrent radiotherapy and chemotherapy starting from day 1 first cycle.
strong recommendation, high quality level of evidence (systematic review, Prospective trial) (24)(25)
Concurrent chemotherapeutic agents
Ifosfamide, etoposide, cyclophosphamide and vincristine should be given with radiotherapy. It is recommended to withhold doxorubicin with radiotherapy and re-institute after completion of radiation.
strong recommendation, high quality level of evidence (Randomised trial, systematic review) (13)(26)
5- Treatment of recurrent/relapsed Ewing Sarcoma
Chemotherapy
Recommended chemotherapy combination
· Irinotecan and temozolomide in 21-day interval cycles, Or
· Ifosfamide, carboplatin and etoposide (if > 6 months).
strong recommendation, high quality level of evidence (prospective clinical trials) (27-28)
Surgery
Surgical resection of both local and metastatic sites (especially pulmonary) if feasible is recommended.
strong recommendation, high quality level of evidence (prospective observational study, retrospective analysis) (29)(30)
Radiotherapy
Radiation is recommended either definitive or postoperative.
strong recommendation, high quality level of evidence (meta-analysis) (30)
6- Surveillance – Follow up - for Ewing Sarcoma patients
X-ray of the primary site is recommended every 4 months for the first 2 years and as clinically warranted.
strong recommendation, high quality level of evidence (prospective observational study, retrospective analysis) (31)
CT chest every 4 months is the recommended chest imaging in the first 2 years. Chest X-ray is recommended for chest imaging in later years.
strong recommendation, high quality level of evidence (prospective observational study, retrospective analysis) (31)
It is recommended to increase intervals of imaging of primary site and chest after 24 months and annually after 5 years (indefinitely).
strong recommendation, high quality level of evidence (prospective observational study, retrospective analysis) (31)
Clinical indicators for monitoring
· Contrast enhanced MRI of primary site.
· CT chest.
· Confirmed Pathology.
· 2 weeks interval between cycles.
· local control after 9 weeks of chemotherapy.
· Radiotherapy referral.
Research Gaps
Comparison of outcome in terms of recurrence and toxicity between local
control modalities in Egyptian patients.
Update of this guideline
This guideline will be updated whenever there is new evidence.
- References
1) Gomez-Brouchet, A., Mascard, E., Siegfried, A., de Pinieux, G., Gaspar, N., Bouvier, C., Aubert, S., Marec-Bérard, P., Piperno-Neumann, S., Marie, B., Larousserie, F., Galant, C., Fiorenza, F., Anract, P., Sales de Gauzy, J., & Gouin, F. (2019). Assessment of resection margins in bone sarcoma treated by Neoadjuvant Chemotherapy: Literature review and guidelines of the Bone Group (GROUPOS) of the French sarcoma group and Bone Tumor Study Group (GSF-geto/RESOS). Orthopaedics & Traumatology: Surgery & Research, 105(4), 773–780.
2) Kissane JM, Askin FB, Foulkes M, et al. Ewing's sarcoma of bone: clinicopathologic aspects of 303 cases from the Intergroup Ewing's Sarcoma Study. Hum Pathol 1983; 14:773-779.
3) Spector, L. G., Hubbard, A. K., Diessner, B. J., Machiela, M. J., Webber, B. R., & Schiffman, J. D. (2021). Comparative international incidence of Ewing Sarcoma 1988 to 2012. International Journal of Cancer, 149(5), 1054–1066.
4) Treglia G, Salsano M, Stefanelli A, et al. Diagnostic accuracy of (1)(8)F-FDG-PET and PET/CT in patients with Ewing sarcoma family tumours: a systematic review and a meta-analysis. Skeletal Radiol 2012; 41:249-256.
5) Siegel MJ, Acharyya S, Hoffer FA, et al. Whole-body MR imaging for staging of malignant tumors in pediatric patients: results of the American College of Radiology Imaging Network 6660 Trial. Radiology 2013; 266:599-609.
6) Boddu S, Walko CM, Bienasz S, et al. Clinical Utility of Genomic Profiling in the Treatment of Advanced Sarcomas: A Single-Center Experience. JCO Precision Oncology 2018:1-8.
7) Campbell KM, Shulman DS, Grier HE, DuBois SG. Role of bone marrow biopsy for staging new patients with Ewing sarcoma: A systematic review. Pediatr Blood Cancer 2021;68: e28807.
8) Nesbit ME, Gehan EA, Burgert EO, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing's sarcoma of bone: a long- term follow-up of the First Intergroup study. J Clin Oncol 1990; 8:1664- 1674.
9) Burgert EO, Nesbit ME, Garnsey LA, et al. Multimodal therapy for the management of nonpelvic, localized Ewing's sarcoma of bone: intergroup study IESS-II. J Clin Oncol 1990; 8:1514-1524.
10) Kolb EA, Kushner BH, Gorlick R, et al. Long-term event-free survival after intensive chemotherapy for Ewing's family of tumors in children and young adults. J Clin Oncol 2003; 21:3423-3430.
11) Shamberger RC, LaQuaglia MP, Gebhardt MC, et al. Ewing sarcoma/primitive neuroectodermal tumor of the chest wall: impact of initial versus delayed resection on tumor margins, survival, and use of radiation therapy. Ann Surg 2003; 238:563-567; discussion 567-568.
12) Brennan B, Kirton L, Marec-Berard P, et al. Comparison of two chemotherapy regimens in Ewing sarcoma (ES): Overall and subgroup results of the Euro Ewing 2012 randomized trial (EE2012). Journal of Clinical Oncology 2020; 38:11500-11500.
13) Womer RB, West DC, Krailo MD, et al. Randomized Controlled Trial of Interval-Compressed Chemotherapy for the Treatment of Localized Ewing Sarcoma: A Report from the Children's Oncology Group. Journal of Clinical Oncology 2012; 30:4148-4154.
14) Yock TI, Krailo M, Fryer CJ, et al. Local control in pelvic Ewing sarcoma: analysis from INT-0091--a report from the Children's Oncology Group. J Clin Oncol 2006; 24:3838-3843.
15) Dunst J, Jurgens H, Sauer R, et al. Radiation therapy in Ewing's sarcoma: an update of the CESS 86 trial. Int J Radiat Oncol Biol Phys 1995; 32:919-930.
16) Schuck A, Ahrens S, von Schorlemer I, et al. Radiotherapy in Ewing tumors of the vertebrae: treatment results and local relapse analysis of the CESS 81/86 and EICESS 92 trials. Int J Radiat Oncol Biol Phys 2005; 63:1562-1567.
17) DuBois SG, Krailo MD, Gebhardt MC, et al. Comparative evaluation of local control strategies in localized Ewing sarcoma of bone: a report from the Children's Oncology Group. Cancer 2015; 121:467-475.
18) Indelicato DJ, Keole SR, Shahlaee AH, et al. Definitive radiotherapy for ewing tumors of extremities and pelvis: long-term disease control, limb function, and treatment toxicity. Int J Radiat Oncol Biol Phys 2008; 72:871-877.
19) Haeusler J, Ranft A, Boelling T, et al. The value of local treatment in patients with primary, disseminated, multifocal Ewing sarcoma (PDMES). Cancer 2010; 116:443-450.
20) Paulino AC, Mai WY, Teh BS. Radiotherapy in metastatic Ewing sarcoma. Am J Clin Oncol 2013; 36:283-286.
21) Baumann BC, Nagda SN, Kolker JD, et al. Efficacy and safety of stereotactic body radiation therapy for the treatment of pulmonary metastases from sarcoma: A potential alternative to resection. J Surg Oncol 2016; 114:65-69.
22) Bolling T, Schuck A, Paulussen M, et al. Whole lung irradiation in patients with exclusively pulmonary metastases of Ewing tumors. Toxicity analysis and treatment results of the EICESS-92 trial. Strahlenther Onkol 2008; 184:193-197.
23) Casey DL, Wexler LH, Meyers PA, et al. Radiation for bone metastases in Ewing sarcoma and rhabdomyosarcoma. Pediatr Blood Cancer 2015; 62:445-449.
24) Mirzaei, L., Kaal, S. E. J., Schreuder, H. W. B., & Bartels, R. H. M. A. (2015). The neurological compromised spine due to Ewing sarcoma. what first. Neurosurgery, 77(5), 718–725.
25) Boussios, S., Hayward, C., Cooke, D., Zakynthnakis-Kyriakou, N., Tsiouris, A. K., Chatziantoniou, A. A., Kanellos, F. S., & Karathanasi, A. (2018). Spinal Ewing sarcoma debuting with cord compression: Have we discovered the thread of Ariadne? Anticancer Research, 38(10), 5589–5597.
26) Dunst J, Schuck A. Role of radiotherapy in Ewing tumors. Pediatr Blood Cancer 2004; 42:465-470.
27) Raciborska A, Bilska K, Drabko K, et al. Vincristine, irinotecan, and temozolomide in patients with relapsed and refractory Ewing sarcoma. Pediatr Blood Cancer 2013; 60:1621-1625.
28) Van Winkle P, Angiolillo A, Krailo M, Cheung YK, Anderson B, Davenport V, Reaman G, Cairo MS. Ifosfamide, carboplatin, and etoposide (ICE) reinduction chemotherapy in a large cohort of children and adolescents with recurrent/refractory sarcoma: the Children's Cancer Group (CCG) experience. Pediatr Blood Cancer. 2005 Apr;44(4):338-47.
29) Xue R, Lewis VO, Moon BS, Lin PP. Local recurrence of Ewing sarcoma: Is wide excision an acceptable treatment? J Surg Oncol 2019; 120:746-752.
30) Robinson SI, Ahmed SK, Okuno SH, et al. Clinical outcomes of adult patients with relapsed Ewing sarcoma: a 30-year single-institution experience. Am J Clin Oncol 2014; 37:585-591.
31) Bacci G, Forni C, Longhi A, et al. Long-term outcome for patients with non-metastatic Ewing's sarcoma treated with adjuvant and neoadjuvant chemotherapies. 402 patients treated at Rizzoli between 1972 and 1992. Eur J Cancer 2004; 40:73-83.