Paediatric Aggressive Mature B Cell Non-Hodgkin Lymphoma (Burkitt lymphoma)
| Site: | EHC | Egyptian Health Council |
| Course: | Pediatric Oncology Guidelines |
| Book: | Paediatric Aggressive Mature B Cell Non-Hodgkin Lymphoma (Burkitt lymphoma) |
| Printed by: | Guest user |
| Date: | Monday, 23 December 2024, 10:27 PM |
Description
"last update: 14 Oct 2024"
- Acknowledgment
We would like to acknowledge the Guidelines Development Group (GDG) of Paediatric Oncology for adapting this Guideline.
• Chair of the Committee:
Prof Alaa El-Haddad (Professor of Paediatric Oncology and Former Dean of the National Cancer Institute - Cairo University, Head of the Paediatric Oncology Department and the Bone Marrow Transplant Unit at the Children’s Cancer Hospital Cairo, Egypt).
• The Scientific Committee Members:
Prof Iman Sidhom (Professor and Head of Department of Paediatric Oncology - National Cancer Institute - Cairo University).
Prof Emad Ebied (Deputy Director, National Cancer Institute and Professor of Paediatric Oncology - National Cancer Institute - Cairo University).
Prof Mahmoud Hammad (Committee Rapporteur and Professor of Paediatric Oncology - National Cancer Institute - Cairo University - and Director of the Oncology and Nuclear Medicine Centre at Nasser Institute Hospital).
Prof Youssef Madney (Professor of Paediatric Oncology - National Cancer Institute - Cairo University - and Consultant of Paediatric Oncology at Dar Al Salam Cancer Hospital Harmal).
Dr Ahmed Mustafa (Lecturer of Paediatric Oncology - National Cancer Institute - Cairo University - and Assistant Head of the Specialized Medical Centres Secretariat for Oncology Affairs).
Dr Zaki Ahmed Zaki (Consultant and Director, Haematology Unit at Sheikh Zayed Specialized Hospital).
Dr Amal Ahmed Zein (Paediatric Oncology Consultant at Al-Sahel Teaching Hospital- and at Oncology Centre of Nasser Institute Hospital).
Prof Shady Fadel (Assistant Professor, Faculty of Medicine, Alexandria University - Director and Head, Paediatric Oncology Department, Borg El Arab Hospital).
Dr Mahmoud Motaz (Lecturer, South Egypt Oncology Institute, Assiut University, Medical Director and Head of the Paediatric Oncology Department at Shifa Al Orman Oncology Centre).
Dr Ebtehal Mahmoud Ali (Assistant lecturer of Paediatric Oncology at National Cancer Institute – Cairo University).
Dr Mai Mamdouh (Clinical Pharmacist, Paediatric Oncology, Dar El Salam Cancer Hospital Harmal).
- Abbreviations
BM (bone marrow)
CNS (central nervous system)
COG (Children’s Oncology Group)
COP (cyclophosphamide, vincristine, prednisone)
COPAD (cyclophosphamide, vincristine, prednisone, and doxorubicin)
COPADM3 (cyclophosphamide, vincristine, prednisone, doxorubicin, and 3-gram methotrexate)
COPADM8 (cyclophosphamide, vincristine, prednisone, doxorubicin, and 8-gram methotrexate)
CR (complete remission)
CRb (complete response biopsy negative)
CRu (complete response unconfirmed)
CSF (cerebrospinal fluid)
CT (computed tomography)
CYM (cytarabine and methotrexate)
CYVE (Cytarabine, Etoposide)
EFS (event-free survival)
FAB (French American-British)
FDG (fluorodeoxyglucose F18)
IHC (immunohistochemistry)
IT (intrathecal)
LDH (lactate dehydrogenase)
LMB (Lymphoma Malignancy B)
LN (lymph node)
MR (minor response)
MRI (magnetic resonance imaging)
NCCN (National Comprehensive Cancer Network)
NHL (non-Hodgkin lymphoma)
NR (no response)
PD (progressive disease)
PET (positron emission tomography)
PR (partial response)
RT (radiation therapy)
SPD (sum of product of greatest perpendicular diameters)
ULN (upper limit of normal)
- Glossary
Sequence 1: Includes prednisolone, cyclophosphamide, doxorubicin, methotrexate, Vincristine. (1)
Sequence 2: Includes cytarabine and etoposide. (1)
Positive FDG-PET/CT: Residual lesion is Deauville score more than 3. (2)
Negative FDG-PET/CT: Residual lesion is Deauville score < 3. (2)
Risk group definitions (1)
Group A
· Completely resected stage I
or
· Completely resected abdominal stage II
Group B (Low risk)
· Unresected stage I and non-abdominal stage II
or
· stage III with low LDH (≤2 times [ULN])
Group B (High risk)
· Stage III with high LDH (>2 times ULN),
or
· All non-CNS stage IV with bone marrow involvement (<25% lymphoma cells)
· Achieving more than 20% regression after COP
Group C
· Any CNS involvement and/or Bone marrow involvement (≥25% lymphoma cells)
· Achieving less than 20% regression after COP
International Paediatric NHL Response criteria (3):
CR
· Disappearance of all disease
· CT or MRI reveals no residual and no new lesions
· Residual mass pathologically negative for disease BM and CS free of disease pathologically
CRb
· Residual mass with no pathologic evidence of disease from limited or core biopsy; no new lesions by imaging examination
· BM and CSF free of disease pathologically
· No new and/or progressive disease elsewhere
CRu
· Residual mass negative by FDG-PET; no new lesions by imaging examination
· BM and CS free of disease pathologically
· No new and/or progressive disease elsewhere
PR
· At least 50% decrease in SPD on CT or MRI; FDG-PET may be positive (Deauville score 4 or 5 with reduced lesional uptake compared to baseline)
· May have evidence of disease in BM or CSF if present at diagnosis but should have 50% reduction in percentage of lymphoma cells.
· No new and/or progressive disease
MR
· Decrease in SPD >25%, but <50% on CT or MRI
· May have evidence of disease in BM or CS if present at diagnosis, but should have 25% to 50% reduction in percentage of lymphoma cells
· No new and/or progressive disease
NR
· Not meeting CR, PR, MR, or PD criteria
PD
· >25% increase in SPD on CT or MRI; Deauville score 4 or 5 on FDG-PET with increase in lesional uptake from baseline; or new morphologic disease in BM or CSF
- Executive Summary
This guidance provides a data-supported approach to the diagnosis, risk stratification, treatment and follow up of paediatric patients diagnosed with Burkitt lymphoma.
|
Level Of recommendation |
|
|
1-Work up for newly diagnosed NHL |
|
|
Pathology specimen is recommended with the proper IHC. |
Strong Recommendation |
|
We recommend whole body FDG- PET CT if available otherwise contrast enhanced CT neck, chest, abdomen and pelvis is recommended. |
Strong Recommendation |
|
Bilateral bone marrow aspiration and biopsy is recommended as well as CSF examination. |
Strong Recommendation |
|
2-Treatment of clinical group A |
|
|
Two 21-day cycles COPAD are recommended. |
Strong Recommendation |
|
Response assessment is recommended to include imaging studies of primary tumour site. |
Strong Recommendation |
|
|
|
|
Multiagent chemotherapy is recommended starting with pre phase COP Followed by response assessment post COP Then administer 2 induction courses COPADM3 and two consolidation courses CYM. |
Strong Recommendation |
|
Contrast enhanced CT neck, chest, abdomen and pelvis is recommended for response assessment after COP and FDG- PET/CT is recommended for assessment after CYM I. |
Strong Recommendation |
|
Response assessment after CYM I: · If in CR, then continuation of CYM II is recommended. · If not in CR, biopsy is recommended. If biopsy is not feasible then continue as group B if PET/CT is negative. Upgrade to group C if biopsy is viable or PET/CT is positive. |
Strong Recommendation |
|
Rituximab addition to chemotherapy is recommended in all high-risk group B |
Strong Recommendation |
|
4-Treatment of clinical group C |
|
|
Multiagent chemotherapy should be initiated with pre phase (R-COP), followed by 2 induction courses (R-COPADM8), 2 consolidation courses (R-CYVE) and 2 maintenance courses (Sequences 1 and 2) |
Strong Recommendation |
|
Rituximab addition to chemotherapy is recommended for all group C patients |
Strong Recommendation |
|
For Group C CNS disease: We recommend a total of 3 intrathecals in pre phase COP and high dose methotrexate after CYVE1 |
Strong Recommendation |
|
5-End of treatment evaluation |
|
|
End of treatment evaluation should be done and if in CR then follow up is recommended. If not in CR, repeat biopsy from suspicious lesions, and if relapse is confirmed, start relapse protocol. |
Strong Recommendation |
|
6-Treatment of relapse or refractory disease |
|
|
Combination chemotherapy is recommended with regimen: (R-ICE) Rituximab, ifosfamide, carboplatin, etoposide and intrathecal chemotherapy |
Strong Recommendation
|
|
7- Surveillance (follow up after end of treatment) |
|
|
Routine scans are not recommended unless clinically suspicious. Monthly clinical examination is recommended for the first 3 years then annually. |
Strong Recommendation |
- Introduction
NHL accounts for approximately 8–10% of all paediatric malignancies and comprises a heterogeneous group of lymphoid neoplasms with diverse clinicopathological and biological profiles. Burkitt lymphoma constitutes around 40% of paediatric NHL cases in developed countries. Despite its aggressive behaviour and high-grade pathology, paediatric survival rates for BL have markedly improved due to significant advances in multi-agent chemotherapy regimens and enhanced supportive care protocols. In high-resource settings, the event-free survival (EFS) now ranges from 80-90%, depending on both the stage of the disease at diagnosis and the primary anatomical site of involvement. (4)(5)
- Scope and purpose
This guideline was developed aiming to enhance the quality of care for paediatric Burkitt lymphoma (NHL) patients by establishing a consistent standard of care nationwide. They focus on aiding in the early diagnosis, treatment, and follow-up of Burkitt lymphoma to achieve better clinical outcomes.
➡️Target audienceClinicians who are involved in the care and treatment of patients with Burkitt lymphoma (NHL), including paediatric oncologists, surgeons, radiologists, pathologists, and palliative care specialists.
- Methodology
A comprehensive search for guidelines was undertaken to identify the most
relevant guidelines to consider for adaptation.
◾ inclusion/exclusion criteria followed in the search and retrieval of
guidelines to be adapted:
- Selecting only evidence-based guidelines (guideline must include a
report on systematic literature searches and explicit links between
individual recommendations and their supporting evidence).
- Selecting only national and/or international guidelines.
- Specific range of dates for publication (using Guidelines published or
updated 2015 and later).
- Selecting peer reviewed publications only.
- Selecting guidelines written in English language.
- Excluding guidelines written by a single author not on behalf of an
organization in order to be valid and comprehensive, a guideline
ideally requires multidisciplinary input.
- Excluding guidelines published without references as the panel needs
to know whether a thorough literature review was conducted and
whether current evidence was used in the preparation of the
recommendations.
◾ All retrieved Guidelines were screened and appraised using AGREE II
instrument (www.agreetrust.org) by at least two members. the panel decided
a cut-off points or rank the guidelines (any guideline scoring above 50% on
the rigour dimension was retained)
The NCCN guidelines are the main source used while formulating the national guidelines for Burkitt lymphoma (NHL).
◾ Evidence assessment
According to WHO handbook for Guidelines we used the GRADE (Grading
of Recommendations, Assessment, Development and Evaluation) approach
to assess the quality of a body of evidence, develop and report
recommendations. GRADE methods are used by WHO because these
represent internationally agreed standards for making transparent
recommendations. Detailed information on GRADE is available through the
on the following sites:
. GRADE working group: http://www.gradeworkingroup.org
. GRADE online training modules: http://cebgrade.mcmaster.ca/
. GRADE profile software: http://ims.cochrane.org/revman/gradepro
◾ Table 1: Quality of evidence in GRADE
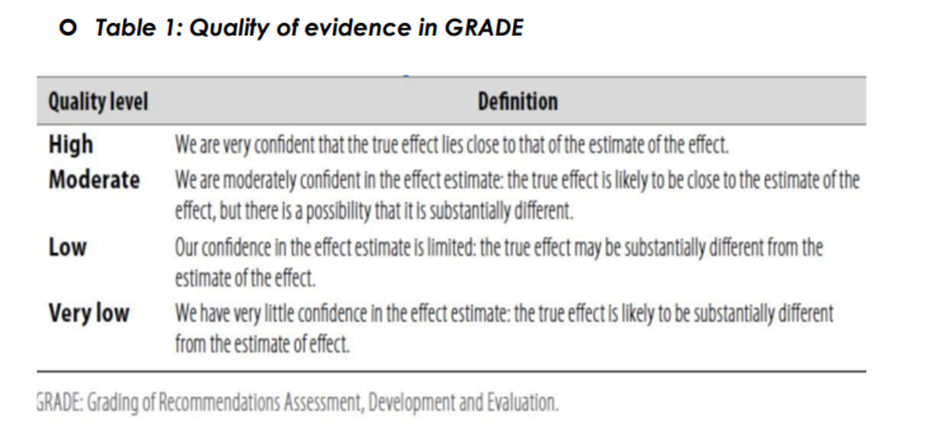
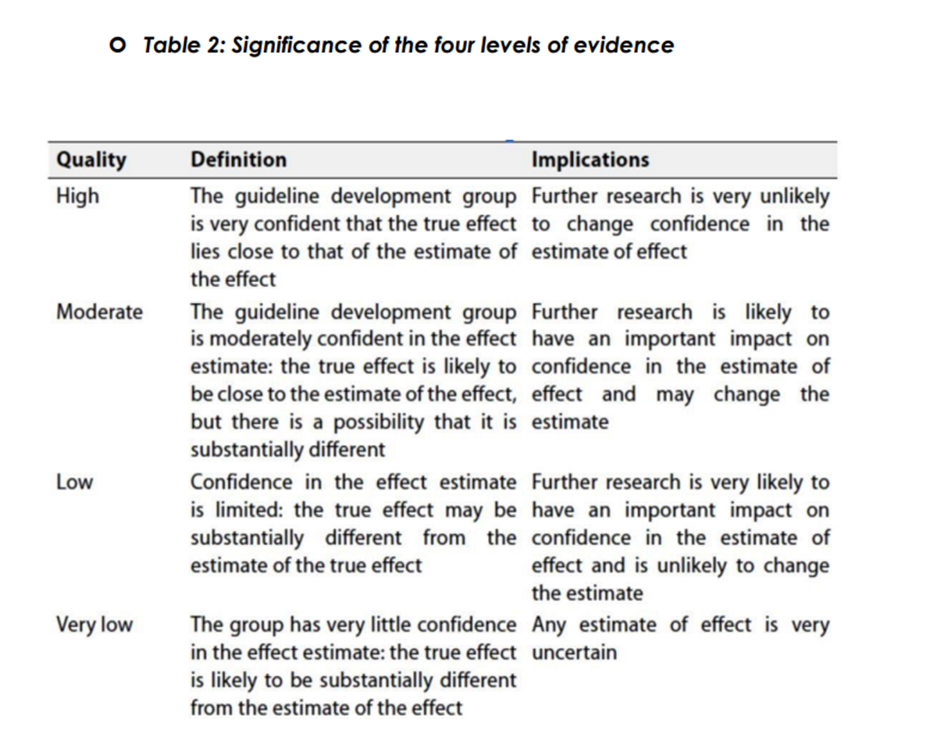
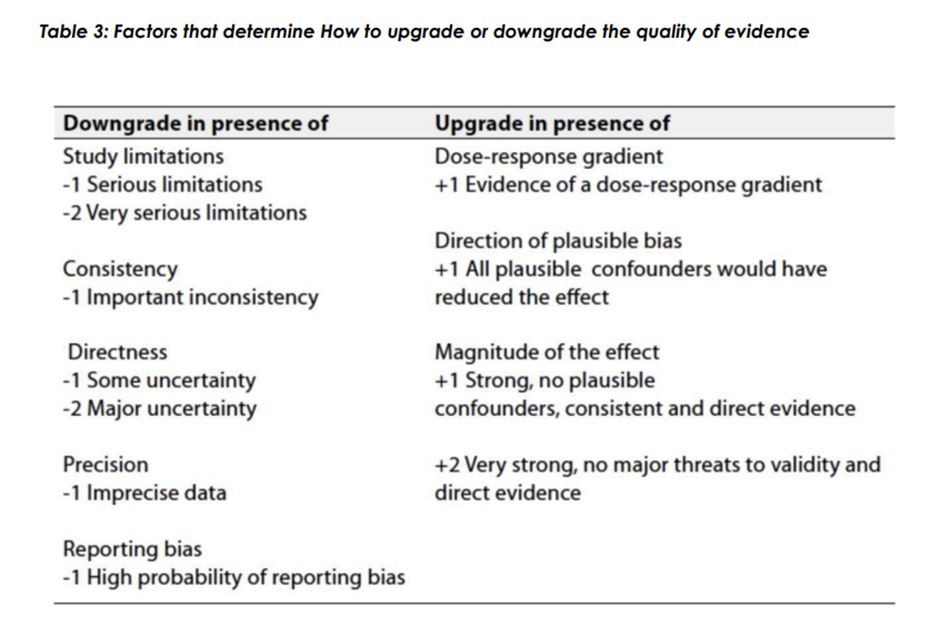
The strength of the recommendation
The strength of a recommendation communicates the importance of adherence to the recommendation:
➡️Strong recommendations
With strong recommendations, the guideline communicates the message that
the desirable effects of adherence to the recommendation outweigh the
undesirable effects. This means that in most situations the recommendation
can be adopted as policy.
➡️Conditional recommendations
These are made when there is greater uncertainty about the four factors
above or if local adaptation must account for a greater variety in values and
preferences, or when resource use makes the intervention suitable for some,
but not for other locations. This means that there is a need for substantial
debate and involvement of stakeholders before this recommendation can be
adopted as policy.
When not to make recommendations.
When there is lack of evidence on the effectiveness of an intervention, it may
be appropriate not to make a recommendation.
- Recommendations
1-Work up for newly diagnosed NHL
Pathology specimen is recommended with the proper IHC.
strong recommendation, high quality level of evidence (ICC report) (6)
We recommend whole body FDG- PET CT if available otherwise contrast enhanced CT neck, chest, abdomen and pelvis is recommended.
strong recommendation, high quality level of evidence (COG report, retrospective analysis, retrospective analysis,) (7)(8)(9)(10)
Bilateral bone marrow aspiration and biopsy is recommended as well as CSF examination.
strong recommendation, high quality level of evidence (prospective trial, retrospective review) (11)(12)
2-Treatment of clinical group A
Two 21-day cycles COPAD are recommended
strong recommendation, high quality level of evidence (prospective trials, multinational cooperative trial) (13)(14)(15)
Response assessment is recommended to include imaging of the primary site.
strong recommendation, high quality level of evidence (COG report, retrospective analysis) (7)(9)
3-Treatment of clinical group B
Multiagent chemotherapy is recommended starting with pre phase COP Followed by response assessment post COP. Then administer 2 induction courses COPADM3 and two consolidation courses CYM.
strong recommendation, high quality level of evidence (prospective trial, prospective randomised trial) (11)(14)
Contrast enhanced CT neck, chest, abdomen and pelvis is recommended for response assessment after COP and FDG- PET/CT is recommended for assessment after CYM I.
strong recommendation, high quality level of evidence (prospective trial, prospective randomised trial) (10)(12)
Response assessment after CYM I:
· If in CR, then continuation of CYM II is recommended.
· If not in CR, biopsy is recommended. If biopsy is not feasible then continue as group B if PET/CT is negative. Upgrade to group C if biopsy is viable or PET/CT is positive.
strong recommendation, high quality level of evidence (prospective trial, prospective randomised trial) (11)(14)
Rituximab addition to chemotherapy is recommended in all high-risk group B
strong recommendation, high quality level of evidence (international prospective randomised trial) (15)
4-Treatment of clinical group C
Multiagent chemotherapy should be initiated with pre phase R-COP, followed by 2 induction courses (R-COPADM8), 2 consolidation courses (R-CYVE) and 2 maintenance courses (Sequences 1 and 2).
strong recommendation, high quality level of evidence (prospective trial, randomised trial) (12)(16)
Rituximab addition to chemotherapy is recommended for all group C patients
strong recommendation, high quality level of evidence (COG report) (17)
For Group C CNS disease: We recommend a total of 3 intrathecals in pre phase COP and high dose methotrexate after CYVE I
strong recommendation, high quality level of evidence (Randomised trial, international randomised trial) (16)(18)
5-End of treatment evaluation
End of treatment evaluation should be done and if in CR then follow up is recommended
If not in CR, repeat biopsy from suspicious lesions, and if relapse is confirmed, start relapse protocol.
strong recommendation, high quality level of evidence (COG report, prospective randomised trial) (7)(12)
6-Treatment of relapse or refractory disease
Combination chemotherapy is recommended with regimen:
(R-ICE) Rituximab, ifosfamide, carboplatin, etoposide and intrathecal chemotherapy
strong recommendation, high quality level of evidence (COG report, retrospective analysis of multicentre trial) (19)(20)
7- Surveillance (follow up after end of treatment)
Routine scans are not recommended unless clinically suspicious. Monthly clinical examination is recommended for the first 3 years then annually.
Conditional recommendation, moderate quality level of evidence (retrospective analysis) (21)
Clinical indicators for monitoring:
· Contrast enhanced CT neck, chest, abdomen and pelvis initially.
· Confirmed Pathology.
· CSF analysis initially.
· Bone marrow aspiration and biopsy.
· Evaluation by imaging of primary site after COP in groups B and C.
· Evaluation after CYM I by imaging of primary site.
Update of this guideline
This guideline will be updated whenever there is new evidence.
- Annexes
Staging of NHL according to international paediatric NHL staging system (22)
Stage I:
· A single tumour not in the mediastinum and abdomen.
Stage II:
· A single extra nodal tumour with regional LN involvement
· Two or more nodal areas on the same side of the diaphragm
· A primary gastrointestinal tract tumour (usually in the ileocecal area, with or without involvement of associated mesenteric nodes, that is completely resectable (if ascites or extension of the tumour to adjacent organs, it should be regarded as stage III)
Stage III:
· Two or more extra nodal tumours (including bone or skin)
· Two or more nodal areas above and below the diaphragm
· Any intrathoracic tumour (mediastinal, hilar, pulmonary, pleural, or thymic)
· Intra-abdominal and retroperitoneal disease, including liver, spleen, ovary, and/or kidney localizations, regardless of degree of resection.
· Any paraspinal or epidural tumour, whether or not other sites are involved.
· Single bone lesion with concomitant involvement of extra-nodal and/or non-regional nodal sites.
Stage IV:
· Any of the above findings with initial involvement of the CNS, bone marrow, or both.
Stage IV, due to CNS disease is considered if one or more of the following applies
· Any lymphoma cells by cytology in CSF.
· Any CNS tumour mass by imaging.
· Cranial nerve palsy (if not explained by extracranial tumour).
· Clinical spinal cord compression.
· Parameningeal extension: cranial and/or spinal
Stage IV disease, due to bone marrow involvement
Defined by morphologic evidence of any lymphoma cells in a bone marrow aspirate.
- References
1) Patte, C. (2001). The Societe Francaise d’Oncologie pediatrique LMB89 protocol: Highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood, 97(11), 3370–3379. https://doi.org/10.1182/blood.v97.11.3370
2) Barrington, S. F., Mikhaeel, N. G., Kostakoglu, L., Meignan, M., Hutchings, M., Müeller, S. P., Schwartz, L. H., Zucca, E., Fisher, R. I., Trotman, J., Hoekstra, O. S., Hicks, R. J., O'Doherty, M. J., Hustinx, R., Biggi, A., & Cheson, B. D. (2014). Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 32(27), 3048–3058.
3) Sandlund JT, Guillerman RP, Perkins SL, et al. International pediatric non-Hodgkin lymphoma response criteria. J Clin Oncol 2015; 33:2106- 2111.
4) Sherief, A. M., Elsafy, U. R., Abdelkhalek, E. R., Kamal, N. M., Youssef, D. M., & Elbehedy, R. (2014). Disease patterns of pediatric non-Hodgkin Lymphoma: A Study from a developing area in Egypt. Molecular and Clinical Oncology, 3(1), 139–144. https://doi.org/10.3892/mco.2014.425
5) O'Rourke, E., Malone, A., O'Marcaigh, A., Storey, L., Betts, D., McDermott, M., & Smith, O. P. (2020). Burkitt Lymphoma/Leukaemia in Children & Young Adolescents. Irish medical journal, 113(1), 6.
6) Campo E, Jaffe ES, Cook JR, et al. The International Consensus Classification of mature lymphoid neoplasms: A report from the Clinical Advisory Committee. Blood 2022;140:1229-1253.
7) Mhlanga J, Alazraki A, Cho SY, et al. Imaging recommendations in pediatric lymphoma: A COG Diagnostic Imaging Committee/SPR Oncology Committee White Paper. Pediatr Blood Cancer 2022:e29968.
8) Abdel Rahman H, Sedky M, Hamoda A, et al. Role of FDG-PET scan in the management of pediatric mature B cell non-Hodgkin's lymphoma. CCHE experience. J Egypt Natl Canc Inst 2016;28:95-99.
9) Karantanis D, Durski JM, Lowe VJ, et al. 18F-FDG PET and PET/CT in Burkitt's lymphoma. Eur J Radiol 2010;75:e68-73.
10) Bailly C, Eugene T, Couec ML, et al. Prognostic value and clinical impact of (18)FDG-PET in the management of children with Burkitt lymphoma after induction chemotherapy. Front Med (Lausanne) 2014;1:54.
11) Cairo MS, Sposto R, Gerrard M, et al. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (≥ 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin's lymphoma: results of the FAB LMB 96 study. J Clin Oncol 2012;30:387-393.
12) Cairo M, Auperin A, Perkins SL, et al. Overall survival of children and adolescents with mature B cell non-Hodgkin lymphoma who had refractory or relapsed disease during or after treatment with FAB/LMB 96: A report from the FAB/LMB 96 study group. Br J Haematol 2018;182:859-869.
13) Gerrard M, Cairo MS, Weston C, et al. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin lymphoma: results of the FAB/LMB96 international study. Br J Haematol 2008; 141:840-847.
14) Patte C, Auperin A, Gerrard M, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood 2007;109:2773-2780.
15) Minard-Colin V, Aupérin A, Pillon M, et al. Rituximab for high-risk, mature B-cell non-Hodgkin's lymphoma in children. N Engl J Med 2020;382:2207-2219.
16) Frazer JK, Li KJ, Galardy PJ, et al. Excellent outcomes in children and adolescents with CNS(+) Burkitt lymphoma or other mature B-NHL using only intrathecal and systemic chemoimmunotherapy: results from FAB/LMB96 and COG ANHL01P1. Br J Haematol 2019; 185:374-377.
17) Goldman S, Smith L, Galardy P, et al. Rituximab with chemotherapy in children and adolescents with central nervous system and/or bone marrow-positive Burkitt lymphoma/leukaemia: a Children's Oncology Group Report. Br J Haematol 2014;167:394-401.
18) Cairo, M.S., Gerrard, M., Patte, C. et al. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood, 109, 2736–2743.
19) Griffin TC, Weitzman S, Weinstein H, et al. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20+) non-Hodgkin lymphoma and mature B- cell acute lymphoblastic leukemia: a report from the Children's Oncology Group. Pediatr Blood Cancer 2009;52:177-181.
20) Osumi T, Mori T, Fujita N, et al. Relapsed/refractory pediatric B-cell non-Hodgkin lymphoma treated with rituximab combination therapy: A report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Pediatr Blood Cancer 2016;63:1794-1799.
21) Eissa HM, Allen CE, Kamdar K, et al. Pediatric Burkitt's lymphoma and diffuse B-cell lymphoma: are surveillance scans required? Pediatr Hematol Oncol 2014;31:253-257.
22) Rosolen A, Perkins SL, Pinkerton CR, et al. Revised International Pediatric Non-Hodgkin Lymphoma Staging System. J Clin Oncol 2015; 33:2112-2118.