Auditory Brainstem Response (ABR) Testing in Babies
| Site: | EHC | Egyptian Health Council |
| Course: | Otorhinolaryngology, Audiovestibular & Phoniatrics Guidelines |
| Book: | Auditory Brainstem Response (ABR) Testing in Babies |
| Printed by: | Guest user |
| Date: | Monday, 23 December 2024, 10:11 PM |
Description
"last update: 26 August 2024"
- Acknowledgements
Chief Editor: Reda Kamel1
General Secretary: Ahmed Ragab2
General Coordinator: Baliegh Hamdy3
Scientific Board: Ashraf Khaled,4 Mohamed Ghonaim,5 Mahmoud Abdel Aziz,6 Tarek Ghanoum,7 Mahmoud Yousef8
Audiology Chief Manager: Tarek Ghannoun7
Audiology Executive Manager: Iman El-Danasoury9
Assembly board: Mona Mourad,10 Yasmine Hamza,11 Mai EL Ghazaly10
Grading Board (In alphabetical order)
Alaa Abou Setta,12 Abeir Dabbous,7 Mohamed El Badry,14 Iman El-Danasoury,9 Tarek ElDessouky,13 Mai El Gohary,15 Trandil El Mahalawy,16 Reham Elshafie,17 Amira El Shennawy,7 Heba Ghannoum,18 Tarek Ghannoum,7 Enas Hassan,19 Nadia Kamal,9 Radwa Mahmoud,20 Salwa Mahmoud,15 Iman Mostafa,13 Nashwa Nada,16 Abir Omara,15 Mohamed Salama,20 Hesham Samy,14 Somia Tawfik9
Reviewing Board: Nagwa Hazzaa,9 Naema Ismail,20 Soha Mekki21
1Otorhinolaryngology Department, Faculty of Medicine/ Cairo University, 2Otorhinolaryngology Department, Faculty of Medicine/ Menoufia University, 3Otorhinolaryngology Department, Faculty of Medicine/ Minia University, 4Otorhinolaryngology Department, Faculty of Medicine/ Bani-Suef University, 5Otorhinolaryngology Department, Faculty of Medicine/ Mansoura University, 6Otorhinolaryngology Department, Faculty of Medicine/ Tanta University, 7Audiovestibular Unit, Otorhinolaryngology Department, Faculty of Medicine/ Cairo University, 8Phoniatrics Unit, Otorhinolaryngology Department, Faculty of Medicine/ Ain Shams University, 9Audiovestibular Unit, Otorhinolaryngology Department, Faculty of Medicine/ Ain Shams University, 10Audiovestibular Unit, Otorhinolaryngology Department, Faculty of Medicine/ Alexandria University, 11Audiovestibular Unit, Otorhinolaryngology Department, Faculty of Medicine/ University of California, Irvine, 12Audiovestibular Unit, Otorhinolaryngology Department, Faculty of Medicine/ Bani-Suef University, 13Audiovestibular Unit, Otorhinolaryngology Department, Faculty of Medicine/ Suez Canal University, 14Audiovestibular Unit, Otorhinolaryngology Department, Faculty of Medicine/ Minia University, 15Audiovestibular Unit, Otorhinolaryngology Department/ Hearing and Speech Institute, 16Audiovestibular Unit, Otorhinolaryngology Department, Faculty of Medicine/ Tanta University, 17Audiovestibular Unit, Otorhinolaryngology Department, Faculty of Medicine/ Fayoum University, 18Audiovestibular Unit, Otorhinolaryngology Department, Faculty of Medicine/ Helwan University, 19Audiovestibular Unit, Otorhinolaryngology Department, Faculty of Medicine/ Assuit University, 20Audiovestibular Unit, Otorhinolaryngology Department, Faculty of Medicine/ Banha University, 21Audiovestibular Unit, Otorhinolaryngology Department, Faculty of Medicine/ Al Azhar University, 22Audiovestibular Unit, Otorhinolaryngology Department, Faculty of Medicine/ Zagazig University.
Sincere thanks extend to the secretaries: Samar Hussein and Eman Ragab, as well as the editor: Mohamed Salah
- Abbreviations
ABR Auditory Brainstem Response
AC Air-conduction
BC Bone-conduction
BSA British Society of Audiology
CM Cochlear Microphonic
CPG Clinical Practice Guideline
C-R Condensation- Rarefaction
CR Clear Response
dBnHL Decibels normal Hearing Level
GRADE Grading of Recommendations Assessment, Development and Evaluation
Hz Hertz
Inc Inconclusive
i/o input to output
NB Narrow Band
NHSP Newborn Hearing Screening Programme (England)
nV nano Volt
PTA Pure tone Audiometry
RA Response Absent
TB Tone Burst
TP Tone Pip
SNR Signal to noise ratio
SPL Sound pressure level
- Executive Summary
Effective and consistent hearing measurement in babies is important for early intervention.
Of particular importance are guidelines to unify testing, data reporting particularly as universal hearing screening is being implemented in EGYPT
● Choice of electrodes & application Skin should be gently abraded. Appropriate options include abrasive electrode paste and cleaning stick with soft cotton material. Single use disposable electrodes are recommended. (Strong recommendation).
● Sedation is not necessary in babies under 12 weeks of age and should be used in babies under 12 months of age only in exceptional circumstances. Sleep deprivation, feeding and diaper change in most situations will lead to natural sleep and lessen activity. (Strong recommendation)
● In order to achieve frequency specificity, thresholds should be measured for at least two frequency audiometric regions:
1. Low frequency thresholds using 0.5 kHz tone bursts or tone pips .
2. High frequency thresholds using 2 or 4 kHz TP / TB or clicks. (Strong recommendation)
● Definition of ABR threshold It is defined (BSA, NHSP 2013) as the lowest level at which a clear response (CR) is present, with a response absent (RA) 5 - 10dB below threshold, under good recording conditions.
● Criteria for ABR response & threshold:
1-Reproducibilty of at least two response traces: visual and graphic.
2-Reproducibility of responses for all intensity i/o functions.
3-Threshold is the reproducible response at the lowest stimulus intensity reached.
(Strong recommendation)
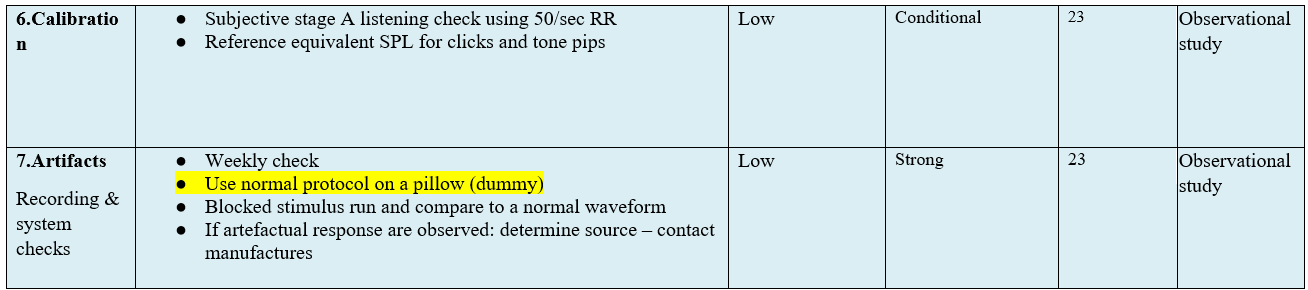
● Calibration :
1. Subjective stage A listening check using 50/sec RR.
2. Reference equivalent SPL for clicks and tone pips if and when feasible.
3. Psychoacoustic calibration control is feasible and easy and can be routinely done.
(Conditional recommendation)
● Reporting:
Results should be clearly marked using the symbols ‘=’, ‘≤’ or ‘<=’, and ‘>’ in addition to the descriptive statements, when important rehabilitation decisions are made.
(Conditional Recommendation)
- Introduction, scope and audience
➡️Introduction
Effective and consistent hearing measurement in babies is important for early intervention. Auditory Brain Response (ABR) is used to identify an accurate estimate of hearing thresholds at different frequencies. It is important to ensure good quality recordings of ABR waveforms which are obtained using earphones, inserts and bone-conduction transducers. As universal hearing screening is being implemented in EGYPT, there is a growing need to unify testing parameters and improve data reporting.
These guidelines are for the use of ABR in assessing hearing in babies up to a corrected age of 12 weeks These guidelines aim at the achievement of the uniformity of the equipment set up, improvement in test performance and waveform interpretation. Frequency-specific information is required. It aims to define criteria by which to identity a ‘clear response’, ‘response absent’ or ‘inconclusive’ response when performing ABR testing in babies.
➡️Target audience:
Audiologists for proper performance and interpretation of ABR. ENT specialists for proper interpretation of ABR results.
- Methods
Methods of development
➡️Stakeholder Involvement: Individuals who were involved in the development process. Included the above-mentioned Audiology Chief Manager, Audiology Executive Manager, Assembly Board, Grading Board and Reviewing Board.
Information about target population experiences were not applicable for this topic.
➡️Search Method
Electronic database searched:
Pubmed, Google scholar, Cochrane and Embase
➡️Keywords
ABR, clicks, tone burst, frequency specific audiological assessment, hearing assessment in babies
The adaptation cycle passed over: set-up phase, adaptation phase (Search and screen, assessment: currency, content, quality & /decision/selection) and finalization phase that included revision and external reviewing.
➡️Time period searched: 2003 to 2019
➡️Results
Three national audiologists reviewed the guidelines available. Guidelines from the British Society of Audiology (BSA) gained the highest scores as regards currency, contents and quality.
It was graded GRADE by twenty one experts and reviewed by three expert reviewers to improve quality, gather feedback on draft recommendations.
The external review was done through a rating scale as well as open-ended questions.
➡️Setting: Primary, secondary and tertiary care centers & hospitals, and related specialties.
Interpretation of strong and conditional recommendations for an intervention
|
Audience |
Strong recommendation |
Conditional recommendation |
|
Patients |
Most individuals in this situation would want the recommended course of action; only a small proportion would not. Formal decision aides are not likely to be needed to help individuals make decisions consistent with their values and preferences. |
Most individuals in this situation would want the suggested course of action, but many would not |
|
Clinicians |
Most individuals should receive the intervention. Adherence to the recommendation could be used as a quality criterion or performance indicator. |
Different choices will be appropriate for individual patients, who will require assistance in arriving at a management decision consistent with his or her values and preferences. Decision aides may be useful in helping individuals make decisions consistent with their values and preferences. |
|
Policymakers |
The recommendation can be adopted as policy in most situations. |
Policy-making will require substantial debate and involvement of various stakeholders. |
WHO handbook for guideline development – 2nd ed.
Chapter 10, page 129
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to Decision frameworks
(GRADE Working Group 2013)
|
Grade |
Definition |
|
High
|
We are very confident that the true effect lies close to that of the estimate of the effect. |
|
Moderate
|
We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
|
Low
|
Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. |
|
Very Low
|
We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect |
- Recommendations
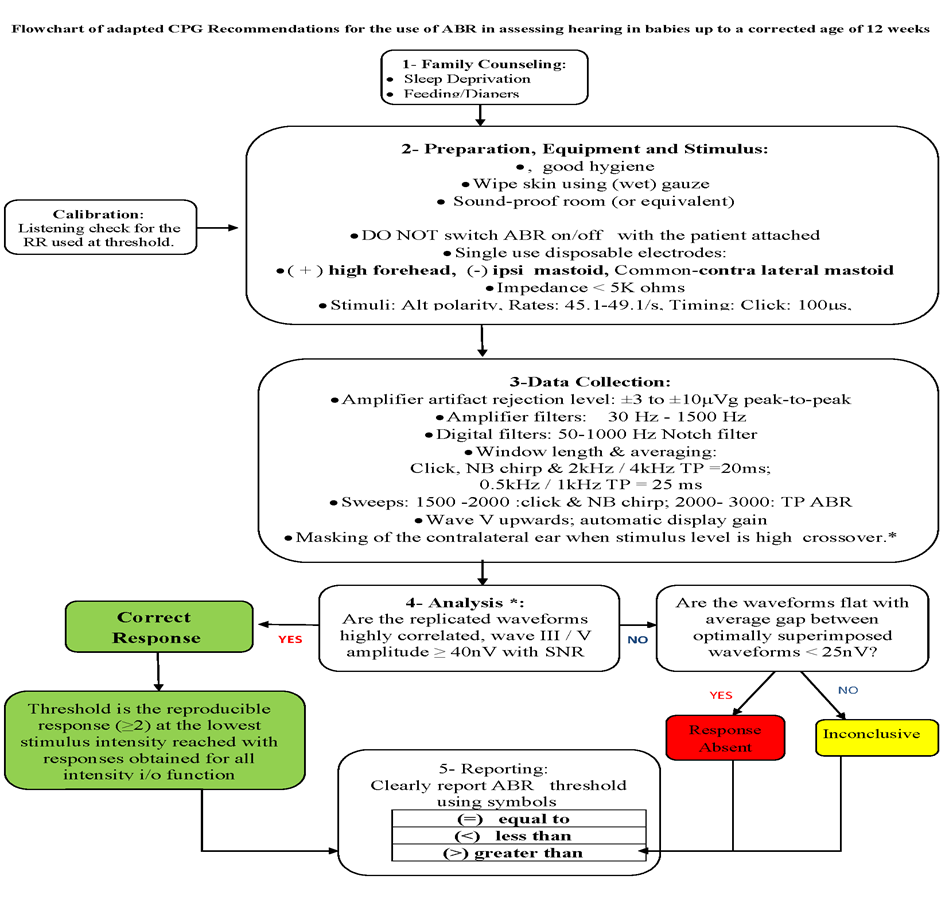
The following statements and flowchart were adapted from the Guidelines from (Auditory Brainstem Response (ABR) Testing in Babies, BSA 2019 due for review 2024) which received the highest scores as regards the currency, contents, and quality.
Recommendations statements
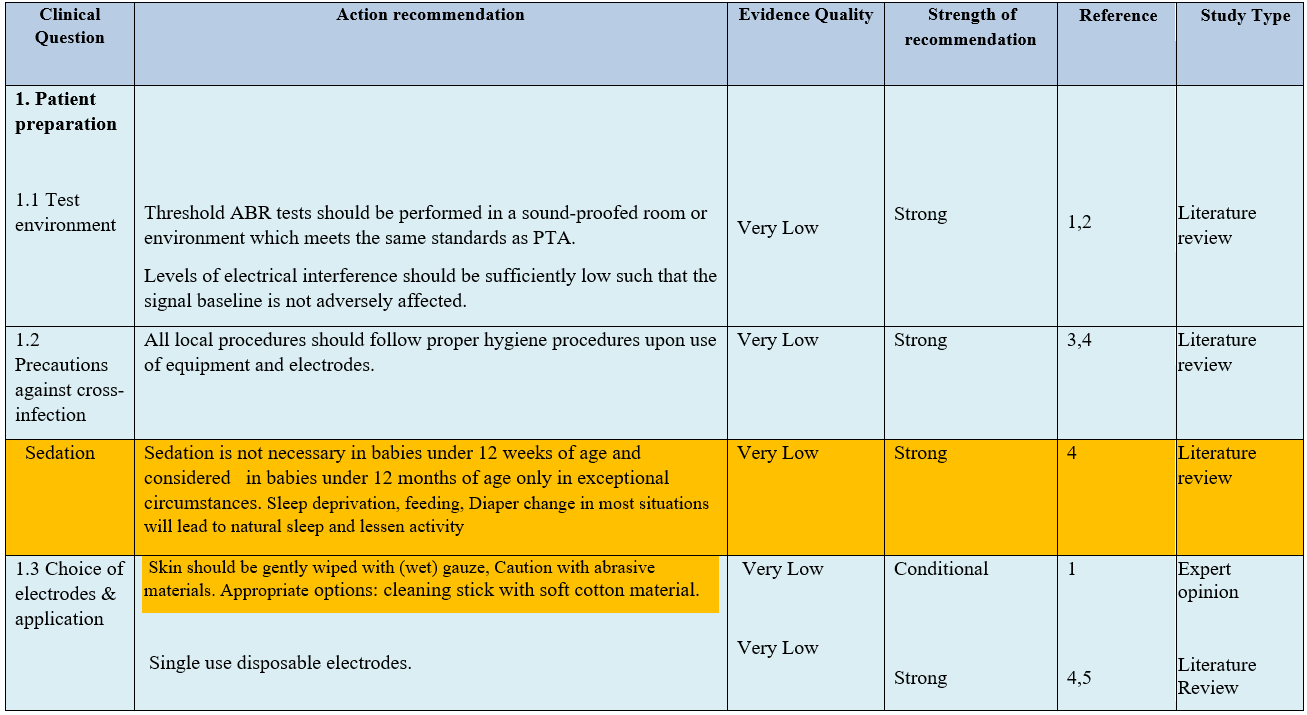
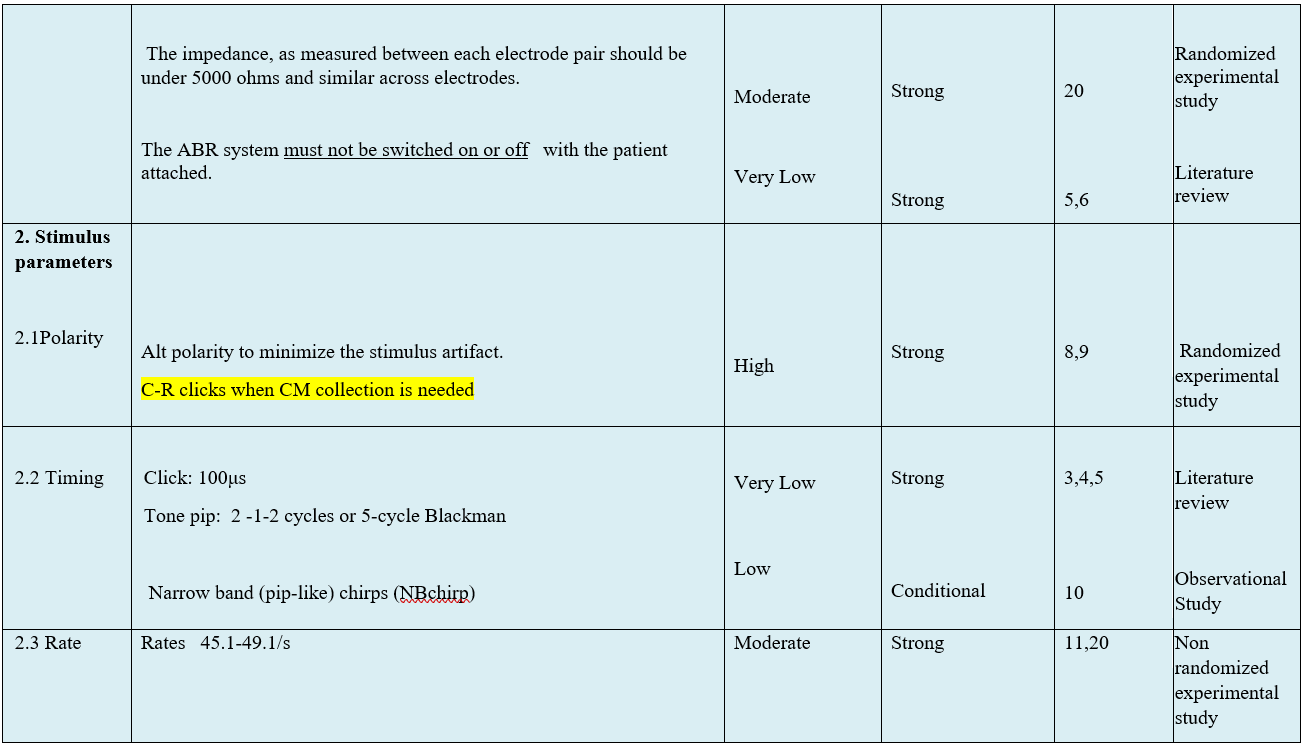
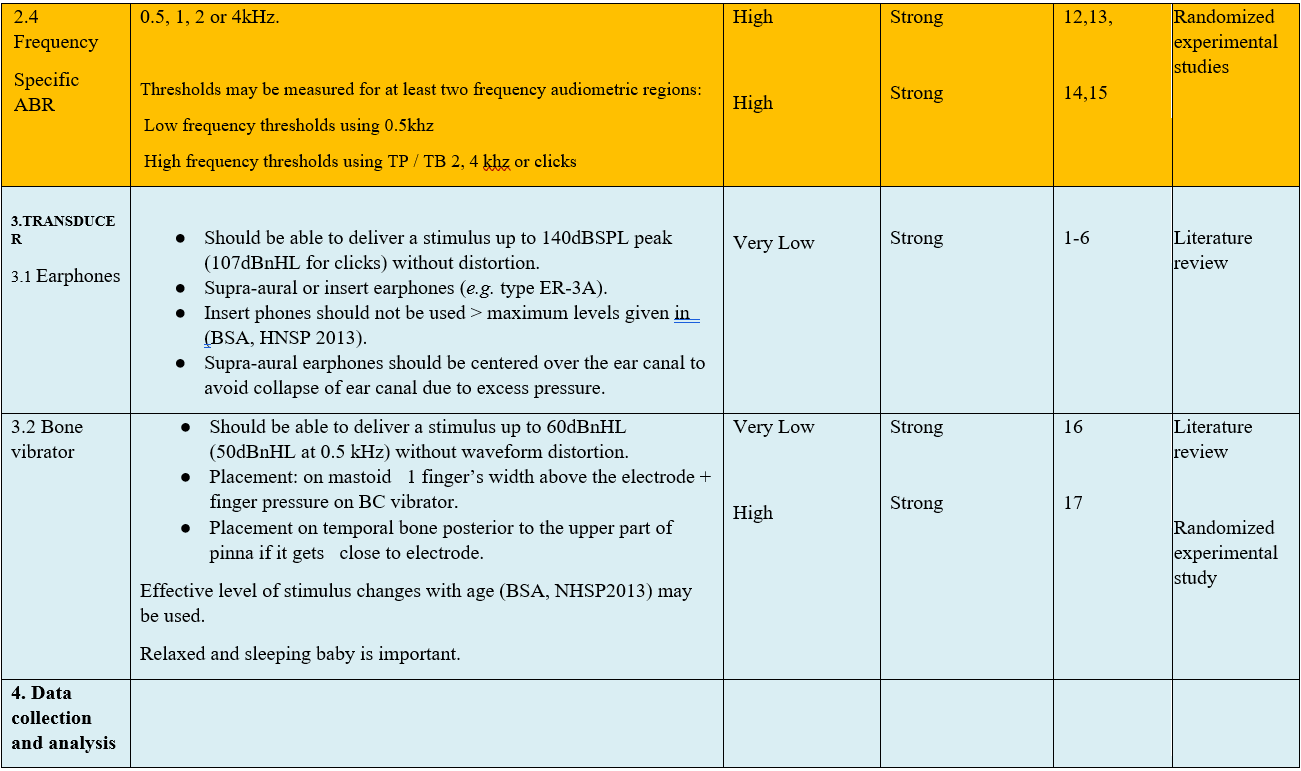
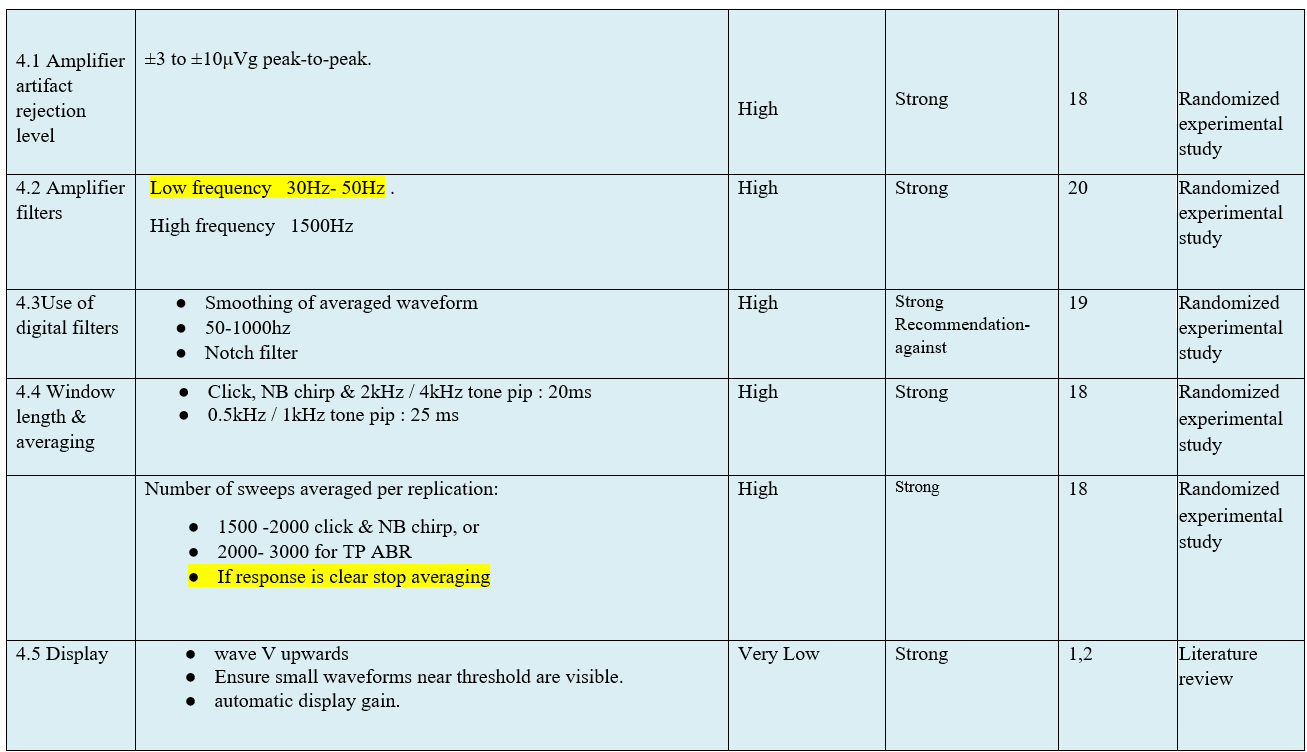
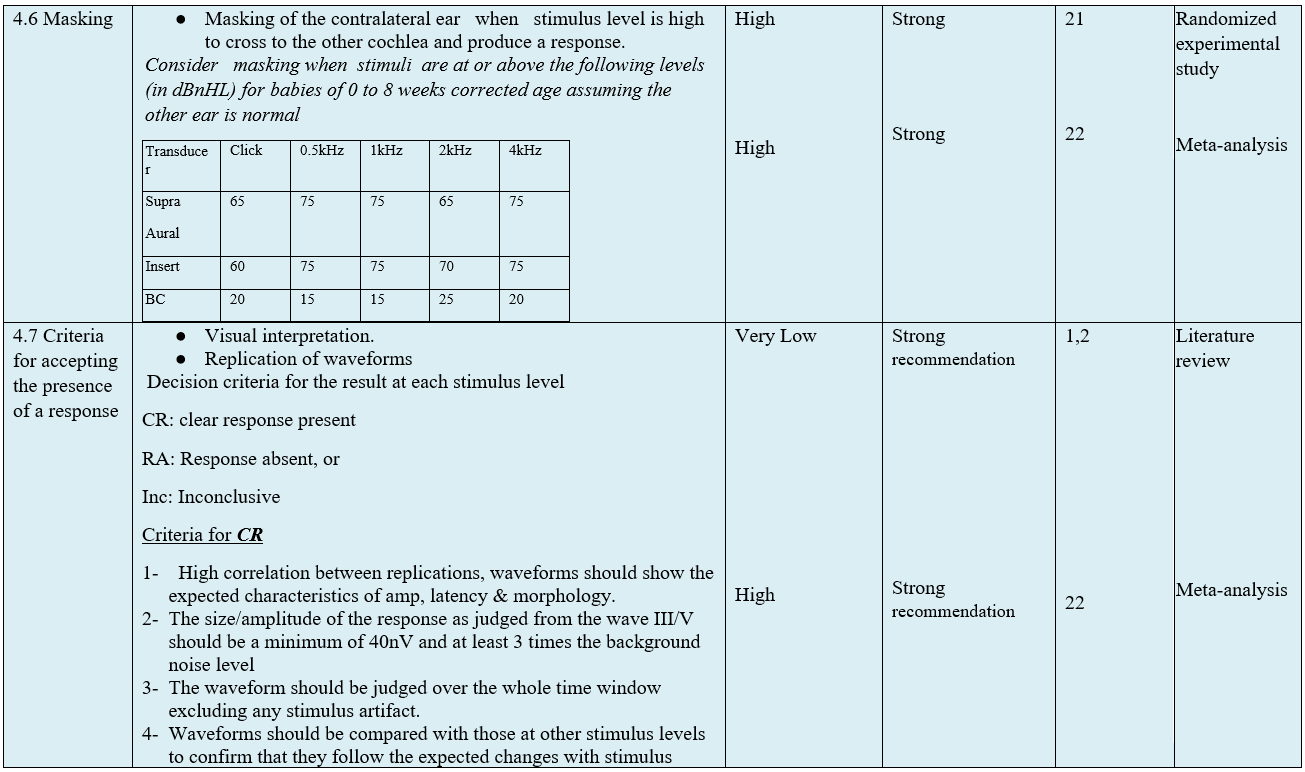
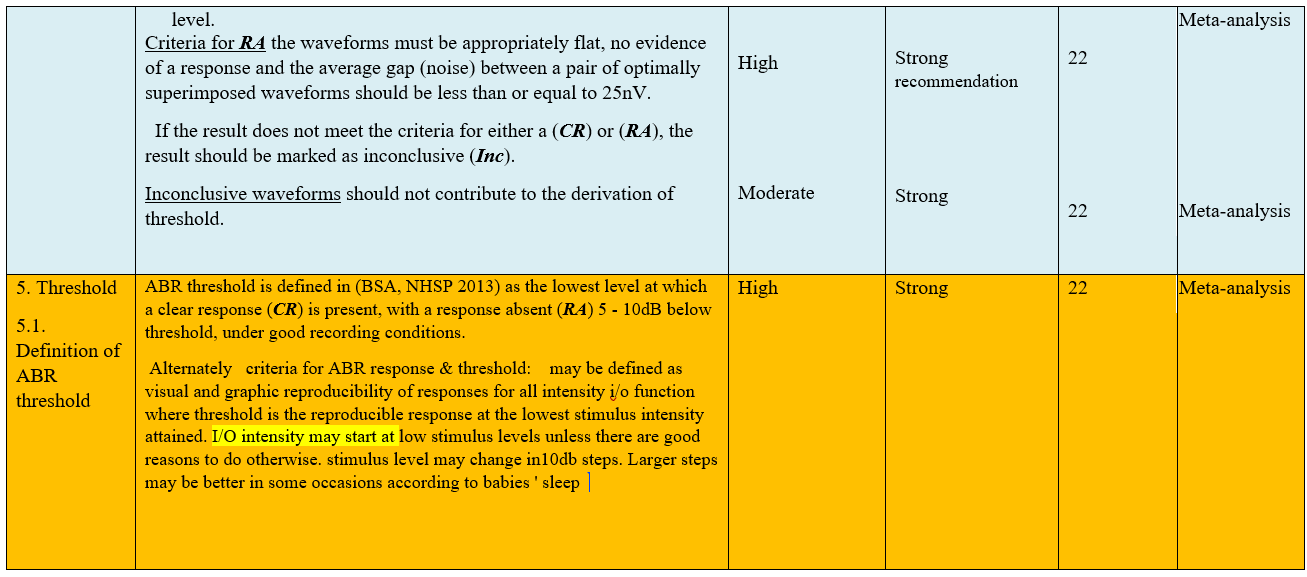
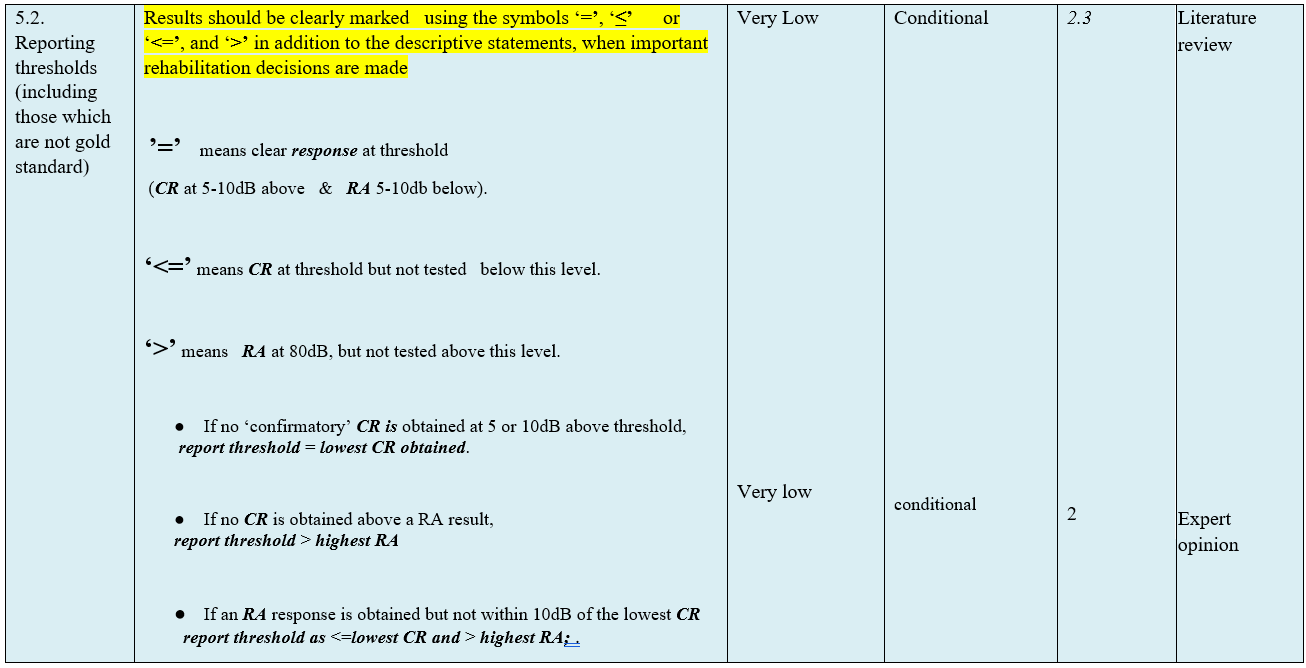
- Research needs
There is a need to conduct randomized controlled trials (RCTs) to determine the efficacy of the short statement versus the full array of ABR testing.
There is a need to conduct RCTs for verification of the efficacy of ABR testing guidelines.
- Monitoring and evaluating the impact of the guideline
Monitoring/ Auditing Criteria: to assess guideline implementation or adherence to recommendations. Clinicians should be able to:
● Acquire a full history from the patient or parents.
● Ensure the acquisition of a two frequency audiogram (low frequency tone burst and high frequency click).
● Ensure the acquisition of two fence intensity regions (low intensity at 30 dBnHL and a higher fence at 60 dBnHL).
● Inform the parents with outcome of ABR test and the follow up needed.
● Give advice on measures needed if hearing loss was reported and the need for repetition at 3 and 6 months of age.
- Updating of the guideline
Updating Procedure:
Any recommendation of this guideline will be updated when new evidence that could potentially impact the current evidence base for this recommendation is identified. If no new reports or information are identified for a particular recommendation, the recommendation will be revalidated. The focus will be on recommendations supported by very-low- or low certainty evidence and where new recommendations or a change in the published recommendations may be needed.
- References
1. American Academy of Audiology. (AAA). Audiologic guidelines for the assessment of hearing in infants and young children. Retrieved July 26, 2017, from https://audiologyweb.s3.amazonaws.com/migrated/201208_AudGuideAssessHear_youth. pdf_5399751b249593.36017703.pdf .2012. from the Newborn Hearing Screening Programme. Version 6 (Wales) October 2013 adapted from version. 2013;3:1.
2. Protocol for auditory brainstem response-based audiological assessment (ABRA) Version 2016.02. https://www.mountsinai.on.ca/care/infant-hearing-program/documents/protocol-for-auditory-brainstem-response-2013-based-audiological-assessement-abra.
3. Year 2019 Position Statement: Principles and Guidelines for early Hearing Detection and Intervention Programs. The Joint Committee on infant hearing. 2019.
4. Hecox K, Galambos R. Brain stem auditory evoked responses in human infants and adults. Archives of otolaryngology. 1974;99(1):30-3.
5. Schulman-Galambos C, Galambos R. Brain stem auditory-evoked responses in premature infants. Journal of Speech and Hearing Research. 1975;18(3):456-65.
6. Mokotoff B, Schulman-Galambos C, Galambos R. Brain stem auditory evoked responses in children. Archives of Otolaryngology. 1977;103(1):38-43.
7. Valenzuela DG, Kumar DS, Atkins CL, Beers A, Kozak FK, Chadha NK. Chloral hydrate sedation for auditory brainstem response (ABR) testing in children: Safety and effectiveness. International journal of pediatric otorhinolaryngology. 2016 Apr;83:175-8. PubMed PMID: 26968073. Epub 2016/03/13. eng.
8. Sininger YS, Masuda A. Effect of click polarity on ABR threshold. Ear and hearing. 1990 Jun;11(3):206-9. PubMed PMID: 2358131. Epub 1990/06/01. eng.
9. Jiang Y, Samuel OW, Asogbon MG, Chen S, Li G. Towards optimal selection of stimuli polarity method for effective evoking auditory brainstem responses. Journal of integrative neuroscience. 2021 Jun 30;20(2):297-305. PubMed PMID: 34258928. Epub 2021/07/15. eng.
10. Ferm I, Lightfoot G, Stevens J. (2013). Comparison of ABR response size, test time and estimation of hearing threshold using frequency specific chirps and tone pips stimuli in newborns. Int J Audiol 52(6): 419-23.
11. Lightfoot, Guy, Yvonne Sininger, Robert Burkard, and Andre Lodwig. 2007. “Stimulus Repetition Rate and The Reference Levels for Clicks and Short Tone B ...” Am J Audiol 16: 94–95.
12. Davis H, Hirsh SK, Popelka GR, Formby C. Frequency selectivity and thresholds of brief stimuli suitable for electric response audiometry. Audiology : official organ of the International Society of Audiology. 1984;23(1):59-74. PubMed PMID: 6704060. Epub 1984/01/01. eng.
13. Davis H, Hirsh SK. The audiometric utility of brain stem responses to low-frequency sounds. Audiology : official organ of the International Society of Audiology. 1976 May-Jun;15(3):181-95. PubMed PMID: 938332. Epub 1976/05/01. eng.
14. Kileny P. The frequency specificity of tone-pip evoked auditory brain stem responses. Ear and hearing. 1981 Nov-Dec;2(6):270-5. PubMed PMID: 7308602. Epub 1981/11/01. eng.
15. Weber BA. Comparison of auditory brain stem response latency norms for premature infants. Ear and hearing. 1982 Sep-Oct;3(5):257-62. PubMed PMID: 7141139. Epub 1982/09/01. eng.
16. Seo YJ, Kwak C, Kim S, Park YA, Park KH, Han W. Update on Bone-Conduction Auditory Brainstem Responses: A Review. Journal of audiology & otology. 2018 Apr;22(2):53-8. PubMed PMID: 29471611. Pubmed Central PMCID: PMC5894486. Epub 2018/02/24. Eng
17. Small, Susan a, Jennifer L Hatton, and David R Stapells. 2007. “Effects of Bone Oscillator Coupling 1725 Method, Placement Location, and Occlusion on Bone-Conduction Auditory Steady-State Responses 1726 in Infants.” Ear and Hearing 28 (1): 83–98. doi:10.1097/01.aud.0000249787.97957.5b. 1727.
18. Lightfoot, G, and J Stevens. 2014. “Effects of Artefact Rejection and Bayesian Weighted Averaging on the Efficiency of Recording the Newborn ABR.” Ear and Hearing 35 (2): 213–20.
19. Lightfoot, Guy, Inga Ferm, Amanda Hall, and Kathryn Evans. 2014. “The Effect of Notch Filtering on the Waveform of the Newborn Auditory Brainstem Response.” International Journal of Audiology 53 (9): 629–32.
20. Stevens, John, Siobhan Brennan, Denise Gratton, and Michael Campbell. 2013. “ABR in Newborns: Effects of Electrode Configuration, Stimulus Rate, and EEG Rejection Levels on Test Efficiency.” International Journal of Audiology 52 (10): 706–12. doi:10.3109/14992027.2013.809482.
21. Weber BA, editor Masking and bone conduction testing in brainstem response audiometry. Seminars in hearing; 1983: Copyright© 1983 by Thieme Medical Publishers, Inc.
22. Stapells, D. R. (2000). Threshold Estimation by the Tone-Evoked Auditory Brainstem Response: A Literature Meta-Analysis. Journal of Speech-Lang Pathology & Audiology, 24, 74-83.
23. NHSP Clinical Group. “Check List for Daily and monthly Function Check of Auditory Brainstem Response systems (stage A check).” 2008.
- Annexes
Editorial Independence:
● This guideline was developed without any external funding.
● All the guideline development group members have declared that they do not have any competing interests.
Annex 1: Guideline Flowchart
Annex 2: Tables of appraisal of selected guidelines: Currency (table 1), Content (table 2) and Quality (table 3) of the selected guidelines.
|
No. |
Guideline Name |
Year of Publication |
The Organization |
Age Demography |
|
1 |
Threshold estimation by the tone evoked auditory brainstem response: A literature meta-analysis |
2000 |
Canadian Journal of Speech-Language Pathology and Audiology BY University of British Columbia |
Adult and infant/ child |
|
2 |
Practice guidelines: Principles of external peer review of auditory electrophysiologic measurements |
2018 |
British Society of Audiology |
Newborn |
|
3 |
Guidelines 9C: Recommended standards for Short latency auditory evoked potentials: American neurophysiological guidelines |
2008 |
American Clinical Neurophysiology Society |
Adult, neonates, infants and children |
|
4 |
Recommended Procedure: Auditory Brainstem Response (ABR) Testing in Babies |
2019 |
British Society of Audiology |
Newborn |
|
5 |
Year 2019 Position Statement: Principles And Guidelines For Early Hearing Detection And Intervention Programs |
2019 |
The Joined Committee On Infant Hearing |
Infants |
|
6 |
Guidelines for the early audiological assessment and management of babies referred from the Newborn Hearing Screening Program: Version 3.1 |
2013 |
The UK NHC and NHS screening programs |
Newborns |
Table 2 Content
|
|
Guideline 1 British Columbia 2000 |
Guideline 2 BSA 2018 |
Guideline 3 American Clinical Neurophysiology Society 2008 |
Guideline 4 BSA 2019 |
Guideline 5 JCIH 2019 |
Guideline 6 NHSP 2013 |
|
Credibility |
7 |
8 |
7 |
9 |
8 |
8 |
|
Observability |
7 |
8 |
8 |
9 |
8 |
8 |
|
Relevance |
8 |
6 |
8 |
9 |
8 |
8 |
|
Relative advantage |
8 |
8 |
7 |
9 |
8 |
7 |
|
Easy to install and understand |
7 |
7 |
8 |
9 |
8 |
8 |
|
Compatibility |
7 |
8 |
7 |
9 |
8 |
7 |
|
Testability |
8 |
5 |
7 |
9 |
8 |
8 |
|
Total score |
48 |
50 |
48 |
72 |
64 |
54 |
Table 3 Quality
|
Domain |
Guideline 1 British Columbia 2000 |
Guideline 2 BSA 2018 |
Guideline 3 American Clinical Neurophysiology Society 2008 |
Guideline 4 BSA 2019 |
Guideline 5 JCIH 2019 |
Guideline 6 NHSP 2013 |
|
Transparency |
A |
A |
A |
A |
A |
A |
|
Conflict of interest |
B |
A |
NR |
A |
B |
A |
|
Development group |
A |
A |
B |
A |
A |
A |
|
Systematic review |
A |
B |
C |
A |
A |
A |
|
Grading of evidence |
B |
A |
B |
A |
B |
B |
|
Recommendations |
A |
A |
A |
A |
A |
A |
|
External review |
B |
B |
C |
A |
A |
B |
|
Updating |
C |
A |
C |
A |
A |
B |
Annex 3: The risks and benefits of added and/or modified statements
|
The statement to be adapted: action |
Benefits |
Risk/Harm |
|
Skin should be gently wiped with (wet) gauze , abrasions avoided |
No possibility of skin injury or skin reaction |
No risk or harm |
|
Sleep deprivation, feeding, Diaper change in most situations will lead to natural sleep and lessen activity |
No possible complications from sedatives No special or additional safety precautions needed |
No risk or harm |
|
Thresholds should be measured for at least two frequency audiometric regions: Low frequency thresholds using 0.5khz High frequency thresholds using TP or TB 2 or 4 khz or clicks |
Allows low & high frequency threshold assessment for two region audiometric representation Reduces test time |
No harm
Potential risk of missing mid frequency HL |
|
Criteria for ABR response & threshold: 1-Reproducibilty of at least two response traces: visual and graphic 2-Reproducibility of responses for all intensity i/o function 3-Threshold is the reproducible response at the lowest stimulus intensity reached |
Simplified criteria Matches all response descriptions in the selected guideline Lessens confusion Allows clear , easy & unified reporting of results |
No harm or risk |
|
Clinician listening check for the RR used at threshold. Reference dBSPLpe/ eq if and when feasible |
Psychoacoustic calibration control is feasible and easy and can be routinely done |
No harm or risk |