BREAST CANCER
| Site: | EHC | Egyptian Health Council |
| Course: | Oncology and Hematological Oncology Guidelines |
| Book: | BREAST CANCER |
| Printed by: | Guest user |
| Date: | Monday, 23 December 2024, 9:36 PM |
Description
"last update: 21 August 2024"
- Acknowledgement
▪️ We would like to acknowledge the Oncology Committee of Egyptian health council (EHC) Guidelines and Breast Cancer Scientific Committee, for adapting this Guidelines.
▪️ Chair of the Committee of Egyptian health council Guidelines: Prof Hussein Khaled.
▪️ The Oncology Committee Members: Ebtesam Saad Eldin, Ehab Khalil, Emad Hamada, Fouad Abuotaleb, Hesham Elghazaly, Hesham Tawfik, Khaled Abdelkarim, Lobna ezz Elarab, Mary Gamal, Mohamed Abdel Mooti, Mohamed Gamil, Nervana Hussein, Ola Khorshid, Omar Sherif Omar, Rasha Fahmi, Rasha Shaltout, Samir Shehata, Yousri Wasef & Yousri Rostom.
▪️ The Breast Cancer Scientific Committee Members: Mohamed Gamil, Lobna Ezz Elarab, Hesham Elghazaly, Ehab Khalil, Rasha Fahmi, Rasha Shaltout, Omar Sherif Omar.
- Abbreviations
AI Aromatase inhibitor
AJCC American joint committee on cancer
Ax Axilla
BC Breast cancer
BCT Breast conserving therapy
BCS Breast conserving surgery
Bx Biopsy
CEM Contrast-enhanced mammography
CT Chemotherapy
EBC Early breast cancer
ECOG The Eastern Cooperative Oncology Group
ESMO European society for medical oncology
ET Endocrine therapy
ER Estrogen receptors
FH Family history
G Gauge
H&E Hematoxylin and eosin
HER2 Human epidermal growth factor receptor2
HR Hormone receptors
IBC Inflammatory BC
IBTR Ipsilateral breast tumor recurrence
IHC Immunohistochemistry
IKWG International Ki67 in Breast Cancer Working Group
IM Internal mammary
ISH In-situ hybridization
LABC Locally advanced breast cancer
LN Lymph nodes
MDT Multi-disciplinary team
Mets Metastasis
MRI Magnetic resonance imaging
MRM Modified radical mastectomy.
NACT Neoadjuvant chemotherapy
NCCN National comprehensive cancer network
NMBC Non metastatic breast cancer
NSM Nipple-sparing mastectomy
OFS Ovarian function suppression
pCR Pathological complete response
PgR Progesterone receptor
PO Per oral
PST Primary systemic therapy
RT Radiotherapy
SC Supraclavicular
SLNB Sentinel LN biopsy
SNM Sono-mammography
SSM Skin-sparing mastectomy
TN Triple negative
TCH Taxotere carboplatin Herceptin
U/S Ultrasound
WLE Wide local excision.
- Glossary
Non-Metastatic Breast Cancer:
Includes patients who have Early Breast Cancer (EBC) and Locally advanced Breast Cancer (LABC).
➡️ EBC: This includes ductal carcinoma in situ and stage I, stage IIA, stage IIB, and stage IIIA breast cancers.
➡️ LABC: this includes inoperable breast adenocarcinoma without distant metastases stage IIIB, and C.
Menopausal status (Defined by NCCN)
Postmenopausal status:
▪️ Permanent cessation of menses includes a profound and permanent decrease in ovarian estrogen synthesis.
Premenopausal status:
▪️ It is the ongoing process of menses and normal ovarian estrogen synthesis.
Perimenopausal status:
▪️ It is the transition status between the pre- and post-menopausal status with irregularities in menses and estrogen levels.
Definitions of molecular subtypes of breast cancer according to: (The ESMO Clinical Practice Guidelines, 2023):
➡️ Luminal A : (ER-positive, HER2-negative, Ki67 low, PgR high)
➡️ Luminal B :
▪️ (HER2-negative subtype): (ER-positive, HER2-negative, and either, Ki67 high or PgR low)
▪️ (HER2-positive subtype): (ER-positive, HER2-positive, any Ki67, any PgR)
➡️ HER2 overexpression (nonluminal): (HER2-positive, ER and PgR absent)
➡️ Triple-negative: (ER and PgR absent, HER2-negative)
Cancers with 1%–100% ER IHC staining are considered ER-positive.
Cancers with 1%–10% ER IHC staining are considered ER-low-positive.
HER2 positive patients (IHC+++ or ISH positive for gene amplification (HER2/CEP17 ratio ≥2.0 AND average HER2 copy number ≥ 4.0 signals/cell))
Ki67 index of 5% or less, or 30% or more, can be used to estimate prognosis for T1-2, N0-1 patients as per the International Ki67 in Breast Cancer Working Group (IKWG)
Definition of High-risk patients:
-HER2-positive disease
-TNBC
- ≥ cT2 or ≥ cN1
-Large primary tumor relative to breast size
- Executive Summary
This guidance provides an evidence-based approach to the diagnosis, staging, treatment and follow up of patients diagnosed with non-metastatic breast cancer (NMBC).
Strength of the recommendation | |
Screening and Early detection (will be discussed in separate guidelines) | |
1.Work up for newly diagnosed breast cancer | |
Laboratory and Radiological Studies We recommend clinical examination of the breasts and regional LNs as well as clinical assessment for distant metastases. | Strong |
Laboratory and Radiological Studies Laboratory assessment should include CBC, renal and liver function tests, alkaline phosphatase and calcium levels, as well as pregnancy test for all women in the childbearing period. | Strong |
Bilateral digital mammography and U/S Examination is the standard imaging for evaluation of both breasts and axillary LNs. | Strong |
Contrast enhanced MRI (or Contrast Mammography) is indicated if Mammography is non-conclusive and in special situations as dense breast invasive lobular carcinoma, axillary lymph node metastasis of unknown primary and in case of suspected multifocality/multicentricity with NACT and BCS planned. | Strong |
Disease stage should be assessed according to the AJCC TNM staging system 8th edition. | Strong |
Imaging of chest, abdomen and bone is recommended for higher-risk patients (locally advanced disease, signs, symptoms, or laboratory results suggesting metastases). | Strong |
FDG-PET-CT is recommended only when conventional methods are inconclusive of metastases. | Conditional |
Initial Biopsy We recommend U/S guided core biopsy for diagnosis of breast masses (at least 4 cores by a 14G needle placed in 10% formalin). | Strong |
We recommend Core biopsy to confirm the diagnosis and histo-pathological type, grade & to evaluate ER, PR, HER-2, KI-67. | Strong |
All patients of child-bearing age should be informed about the effect of CT on fertility and referred to a fertility team when needed | Strong |
2.DCIS | |
DCIS should be preferentially treated with BCS and WBRT or, in cases of extensive or multicentric DCIS, mastectomy should be done. | Strong |
Both tamoxifen and AIs may be used after local BCT for DCIS to prevent local recurrence and to decrease the risk of developing a second primary breast cancer. | Strong |
Following mastectomy for DCIS, tamoxifen or AIs might be considered to decrease the risk of contralateral breast cancer in patients with a high risk of new breast tumors. | Conditional
|
3.Surgery | |
BCS with post-operative RT is strongly recommended as the preferred local treatment option for most patients with EBC. | Strong |
BCT should not be done in case of inflammatory BC, multicentric tumors, pregnancy, history of prior therapeutic radiation therapy (RT) that included a portion of the affected breast, diffuse malignant looking microcalcifications, and diffusely positive margins despite multiple attempts of re-excision. | Strong |
If mastectomy is indicated/preferred, breast reconstruction could be offered, except for primary inflammatory and other high-risk tumors where delays in systemic/radiation treatment would compromise care. | Conditional |
SLNB is the standard axillary surgery in all cN0 patients. | Strong
|
- cN0 - patients with micro metastatic spread or with limited SLN involvement (1-2 affected SLNs), - subsequent whole breast radiotherapy, eventually including the lower part of axilla, and - adjuvant systemic treatment | Strong
|
ALND following positive SLNB with < 3 involved SLNs is generally recommended only in case of expected high axillary disease burden or impact on further adjuvant systemic treatment decisions. | Strong |
Surgical planning following primary systemic therapy should consider the post-PST situation. | Strong |
4. Radiotherapy | |
WBRT is recommended after BCS. | Strong |
Hypo fractionated schedules are recommended: moderate (i.e. 15-16 fractions of < 3 Gy per fraction daily for all indications of post-operative radiotherapy) | Strong |
Ultrahypo fractionated (i.e. 26 Gy in five daily fractions for whole-breast or chest wall, without reconstruction, irradiation) in highly specialized centers | Conditional |
Post mastectomy radiotherapy (PMRT) is recommended for high-risk EBC, including involved resection margins, ≥ 4 involved ALNs, T3-T4 tumors and in the presence of combinations of other risk factors. | Strong |
PMRT should be considered in patients with intermediate-risk features (e.g. lympho vascular invasion, age), including those with 1-3 positive ALNs. | Strong |
5.Adjuvant systemic treatment | |
Strong | |
Hormonal treatment Pre-menopause in Luminal A and B Tamoxifen 20 mg per day PO for 5 years should be the standard of care for low risk patients. If the patient becomes menopausal during or after the first 5 years of TAM, switching to AI is an option. | Strong |
Addition of ovarian function suppression (OFS) to tamoxifen is recommended in patients younger than 40 years with adverse prognostic factors (large tumors T2 or more, positive LNs and high grade). The duration of OFS should be 5 years. | Conditional |
Adjuvant OFS plus an aromatase inhibitor (AI) for 5-10 years is recommended in high-risk patients or in case of contraindications for tamoxifen. The duration of OFS should be 5 years. | Strong |
Post-menopause in Luminal A and B Tamoxifen 20 mg per day for 5 years should be considered only in very low risk patients. Extended adjuvant treatment with tamoxifen up to 10 years is recommended in intermediate and high risk patients with contraindications or intolerance to aromatase inhibitors. | Strong |
Aromatase inhibitors should be part of the adjuvant hormonal treatment of post-menopausal women using one of the following strategies: a) Upfront use for 5 years in high risk patients and those with contraindications to tamoxifen; b) Sequential treatment with 2-3 years of AI after 2 to 3 years of tamoxifen; and c) Extended adjuvant, with 2-5 years of AI after ending first 5 years of adjuvant endocrine therapy should be discussed with all patients with intermediate and high risk disease. | Strong |
Adjuvant chemotherapy in Triple negative, High risk Luminal B negative: Adjuvant chemotherapy is recommended in all patients with triple negative, Her-2 positive and high-risk luminal HER2-negative tumors. | Strong |
Sequential regimen of 4 cycles of anthracycline based chemotherapy then 4 cycles taxane is considered the standard of care. | Strong |
Adjuvant chemotherapy in Her-2 positive, High risk Luminal B HER2-postive: Adjuvant trastuzumab is recommended for all HER2 positive patients (IHC+++ or ISH positive for gene amplification) with invasive tumors >10mm. | Strong
|
We recommend, in Intermediate risk patients (T2-3 or N1 disease) adjuvant trastuzumab every 3 weeks for one year (17 cycles) in combination with taxane based chemotherapy, adjuvant radiotherapy or endocrine therapy. | Conditional
Strong
|
In very high risk patients (T4 or N2-3), adjuvant trastuzumab +/- pertuzumab every 3 weeks is recommended for up to one year (17 cycles) in combination with taxane based chemotherapy, adjuvant radiotherapy or endocrine therapy. | Conditional
|
We recommend the TCH regimen +/- pertuzumab or not according to risk stratification, as a preferred systemic regimen in Her2 positive patients, especially for those with risk factors for cardiac toxicity. | Strong |
6. Neoadjuvant treatment: | |
All intrinsic subtypes: Neoadjuvant therapy is indicated in all inoperable breast cancer (inflammatory BC, T4 or N2-3 disease) to allow operability irrespective of the biological subtype. | Strong |
Neoadjuvant therapy should also be indicated in operable patients to allow for breast conservation and in most patients with aggressive biological subtypes (as triple negative and HER2 positive tumors). | Strong |
HER2 positive subtype: Adding pertuzumab + trastuzumab to taxane based neoadjuvant chemotherapy in HER2 positive patients may be indicated. | Conditional |
TNBC subtype: Adding carboplatin to neoadjuvant taxane based chemotherapy in stage II & III triple negative patients in view of event free survival improvement in addition to higher pCR rate. | Conditional |
Luminal A like subtype special situations: In post-menopausal patients with Luminal A like disease and significant co-morbidities, neoadjuvant endocrine therapy with at least 6 months of AIs can be considered. | Conditional |
Post neoadjuvant therapy Patients with clinical stage cT1-3, N0 who attained pathological complete response (ypT0/is, N0) after neoadjuvant chemotherapy & antiHER2 therapy should complete a year of trastuzumab every 3 weeks for a total of 1 year (17 cycles) of anti-HER2 including neo-adjuvant doses. | Strong |
Patients with clinical stage cT4 or N1-3 who attained pathological complete response (ypT0, is N0) after neoadjuvant chemotherapy & antiHER2 therapy should complete a year of dual blockade with trastuzumab +/- pertuzumab every 3 weeks for a total of 1 year (17 cycles) of anti-HER2 including neo-adjuvant doses. | Conditional |
We recommend, in patients with TNBC who received neoadjuvant chemotherapy and who have a residual invasive disease at the time of surgery, offering adjuvant capecitabine for 6-8 cycles. | Strong |
7. Surveillance: |
|
History and Clinical examination 2-4 times per year for 2 years then every 6 mnths from 3rd to 5th year then annually Annual bilateral (after BCT) or contralateral mammography (after mastectomy) is recommended. | Strong
Strong |
- Introduction
Breast cancer is the most common cancer in females and the second most common in the Egyptian population with more than 26 thousand newly diagnosed cases. (1) Moreover, it is also the second cause of cancer death in Egypt after hepatocellular carcinoma with estimated mortality rate around 10% in 2022. Approximately 46,000 incident cases are forecasted in 2050.
➡️ Purpose and scope
These guidelines will help to improve the quality of care for non-metastatic breast cancer patients via providing a uniform standard of care across the country to help in early diagnosis and treatment for breast cancer, with less aggressive treatment options and improved clinical outcomes. These guidelines cover primary diagnosis, staging, treatment and follow-up of breast cancer patients.
➡️ Target audience
Clinicians who are involved in the care and treatment of patients with breast cancer, including medical oncologists, radiation oncologists, clinical oncologists , surgeons, interventional radiologists, radiologists and pathologists.
- Methodology
A comprehensive search for guidelines was undertaken to identify the most relevant guidelines to consider for adaptation. inclusion/exclusion criteria followed in the search and retrieval of guidelines to be adapted:
- Selecting only evidence-based guidelines (guidelines must include a report on systematic literature searches and explicit links between individual recommendations and their supporting evidence). - Selecting only national and/or international guidelines.
- Specific range of dates for publication (using Guidelines published or updated 2015 and later).
- Selecting peer reviewed publications only.
- Selecting guidelines written in English language.
- Excluding guidelines written by a single author not on behalf of an organization to be valid and comprehensive, a guideline ideally requires multidisciplinary input.
- Excluding guidelines published without references as the panel needs to know whether a thorough literature review was conducted and whether current evidence was used in the preparation of the recommendations.
All retrieved Guidelines were screened and appraised using AGREE II instrument (www.agreetrust.org) by at least two members. the panel decided a cutoff point or rank the guidelines (any guideline scoring above 50% on the rigor dimension was retained)
The NCCN, ESMO, NICE guidelines are the main sources used while formulating the national guidelines for breast cancer.
- Evidence assessment
According to WHO handbook for Guidelines we used the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach to assess the quality of a body of evidence, develop and report recommendations. GRADE methods are used by WHO because these represent internationally agreed standards for making transparent recommendations. Detailed information on GRADE is available through the on the following sites:
▪️ GRADE working group: http://www.gradeworkingroup.org
▪️ GRADE online training modules: http://cebgrade.mcmaster.ca/
▪️ GRADE profile software: http://ims.cochrane.org/revman/gradepro
Table 1: Quality of evidence in GRADE
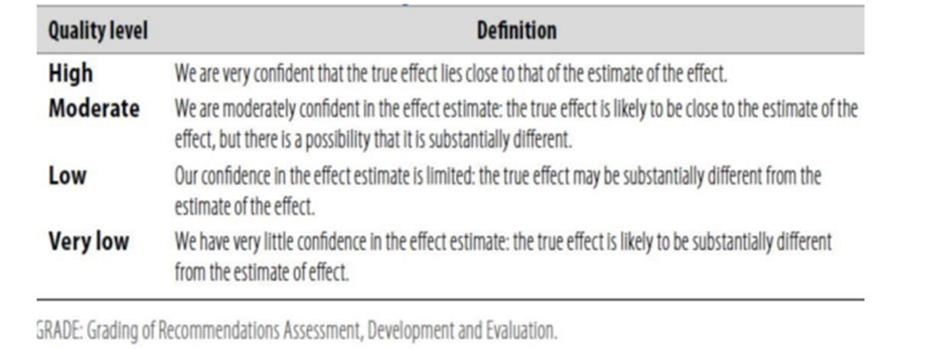
Table 2: Significance of the four levels of evidence

Table 3: Factors that determine how to upgrade or downgrade the quality of evidence.
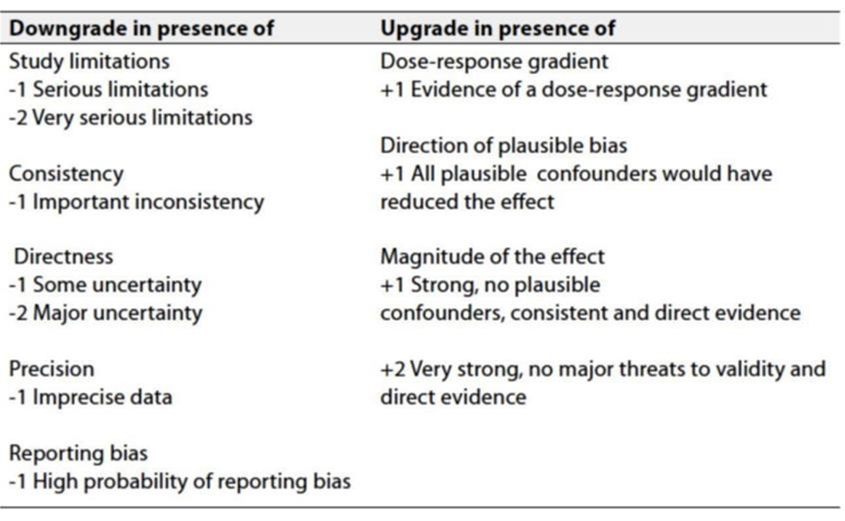
- The strength of the recommendation
The strength of a recommendation communicates the importance of adherence to the recommendation:
➡️Strong recommendations: With strong recommendations, the guideline communicates the message that the desirable effects of adherence to the recommendation outweigh the undesirable effects. This means that in most situations the recommendation can be adopted as policy.
➡️Conditional recommendations: These are made when there is greater uncertainty about the four factors above (Table 2) or if local adaptation must account for a greater variety in values and preferences, or when resource use makes the intervention suitable for some, but not for other locations. This means that there is a need for substantial debate and involvement of stakeholders before this recommendation can be adopted as policy.
When not to make recommendations; when there is lack of evidence on the effectiveness of an intervention, it may be appropriate not to make a recommendation.
- Recommendations
1. Workup for Newly Diagnosed Breast Cancer:
• We recommend clinical examination of the breasts and regional LNs as well as clinical assessment for distant metastases.
Strong recommendation, Moderate quality level of evidence, Narrative Review (2).
• Laboratory assessment should include CBC, renal and liver function tests, alkaline phosphatase and calcium levels, as well as pregnancy test for all women in the childbearing period.
Strong recommendation, Moderate quality level of evidence, Narrative Review (2).
• We recommend bilateral digital mammography and U/S Examination as standard imaging for evaluation of both breasts and LNs.
Strong recommendation, high quality level of evidence, Systemic review (3).
• We recommend Contrast enhanced MRI (or Contrast Mammography) is indicated if Mammography is non-conclusive and in special situations; Dense breast, Invasive lobular carcinoma, Axillary lymph node metastasis of unknown primary and In case of suspected multifocality/multicentricity with NACT and BCS planned.
Strong recommendation, high quality level of evidence meta-analysis (4).
• The disease stage should be assessed according to the AJCC TNM staging system, 8th Edition. “Attached at Annexes “
Strong recommendation, high quality level of evidence, Systemic Review (5).
• We recommend Imaging chest, abdomen, pelvis by CT scan and bone by bone scan for higher-risk patients (locally advanced disease, signs, symptoms, or laboratory results suggesting metastases).
Strong recommendation, Moderate quality level of evidence, Narrative Review (6,7).
• FDG-PET-CT is recommended only when conventional methods are inconclusive of metastasis.
Conditional recommendation, Moderate quality level of evidence, Narrative Review (6,7).
• We recommend U/S guided core biopsy for diagnosis of breast masses (at least 4 cores by a 14G needle placed in 10% formalin) + FNAB from axillary LNs
Strong recommendation, high quality level of evidence, Meta analysis (8, 9).
• We recommend Core biopsy to confirm the diagnosis and histo-pathological type, grade &to evaluate ER, PR, HER-2, KI-67.
Strong recommendation, high quality level of evidence, Systemic Review (10, 11, 12).
• All patients of child-bearing age should be informed about the effect of CT on fertility and referred to a fertility team when needed
Strong recommendation, high quality level of evidence, Randomized Trial (13).
2. DCIS
• DCIS should be preferentially treated with BCS and WBRT or, in cases of extensive or multicentric DCIS, mastectomy should be done.
Strong recommendation, high quality level of evidence, Randomized Trial (14)
• Both tamoxifen and AIs may be used after local BCT for DCIS to prevent local recurrence and to decrease the risk of developing a second primary breast cancer.
Strong recommendation, high quality level of evidence, Randomized Trial (15,16)
• Following mastectomy for DCIS, tamoxifen or AIs might be considered to decrease the risk of contralateral breast cancer in patients with a high risk of new breast tumors.
Conditional, Small, randomized trials or meta-analyses with demonstrated heterogeneity (17)
3. Surgery
• BCS with post-operative RT is strongly recommended as the preferred local treatment option for most patients with EBC.
Strong recommendation, high quality level of evidence, Randomized Trial (18)
• BCT should not be done in case of inflammatory BC, multicentric tumors, pregnancy, history of prior therapeutic radiation therapy (RT) that included a portion of the affected breast, diffuse malignant looking microcalcifications, and diffusely positive margins despite multiple attempts of re-excision.
Strong recommendation, high quality level of evidence, Randomized Trial (18)
• If mastectomy is indicated/preferred, breast reconstruction could be offered, except for primary inflammatory and other high-risk tumors where delays in systemic/radiation treatment would compromise care.
Conditional recommendation, high quality level of evidence, Randomized Trial (19)
• SLNB is the standard axillary surgery in all cN0 patients.
Strong recommendation, high quality level of evidence, Randomized Trial (20)
• Following upfront BCS, further axillary surgery should not be done in cases with the following criteria:
- cN0,
- patients with micro metastatic spread or with limited SLN involvement (1-2
affected SLNs),
- subsequent whole breast radiotherapy,
eventually including the lower part of axilla, and
- adjuvant systemic treatment
Strong recommendation, high quality level of evidence, Randomized Trial (21)
• ALND following positive SLNB with <3 involved SLNs is generally recommended only in case of expected high axillary disease burden or impact on further adjuvant systemic treatment decisions.
Strong recommendation, high quality level of evidence, Randomized Trial (22,23)
• Surgical planning following primary systemic therapy should consider the post-PST situation.
Strong recommendation, high quality level of evidence, Randomized Trial (24,25)
4. Radiotherapy
• WBRT is recommended after BCS.
Strong recommendation, high quality level of evidence, Randomized Trial (26)
• Hypo fractionated schedules are recommended: moderate (i.e. 15-16 fractions of < 3 Gy per fraction daily for all indications of post-operative radiotherapy)
Strong recommendation, high quality level of evidence, Randomized Trial (27,28)
• Ultrahypo fractionated (i.e. 26 Gy in five daily fractions for whole-breast or chest wall, without reconstruction, irradiation).
Conditional recommendation, high quality level of evidence, Randomized Trial (27,28)
• Post mastectomy radiotherapy (PMRT) is recommended for high-risk EBC, including involved resection margins, >4 involved ALNs, T3-T4 tumors and in the presence of combinations of other risk factors.
Strong recommendation, high quality level of evidence, Randomized Trial (29)
• PMRT should be considered in patients with intermediate-risk features (e.g. lympho vascular invasion, age), including those with 1-3 positive ALNs (31).
Strong recommendation, high quality level of evidence, Randomized Trial (29)
• Several factors should be combined for decision of adjuvant systemic therapy as tumor size, lymph node status, lympho-vascular invasion, tumor grade, HR status, HER-2, Ki-67, age, ECOG performance status and comorbidities, and should be discussed in an MDT setting between different subspecialities.
Adjuvant Endocrine Treatment.
Strong recommendation, high quality level of evidence, Randomized Trial (30,31)
(In Adjuvant Endocrine Treatment (Luminal A and B), Premenopausal):
• Tamoxifen 20 mg per day PO for 5 years should be the standard of care for low risk patients. If the patient becomes postmenopausal during or after the first 5 years of TAM, switching to AI is an option.
Strong recommendation, high quality level of evidence, Phase III Randomized Trial (30).
• Addition of ovarian function suppression (OFS) to tamoxifen is recommended in patients younger than 40 years with adverse prognostic factors (T2 or more, positive LNs and high grade). The duration of OFS is 5 years.
Strong recommendation, high quality level of evidence, Phase III Randomized Trial (31)
• Adjuvant OFS plus an aromatase inhibitor (AI) for 5-10 years is recommended in high-risk patients or in case of contraindications for tamoxifen. The duration of OFS is 5 years.
Strong recommendation, high quality level of evidence, Meta-analysis (32)
(Adjuvant Endocrine Treatment (Luminal A and B), Postmenopausal):
• Tamoxifen 20 mg per day for 5 years should be considered only in very low risk patients. Extended adjuvant treatment with tamoxifen up to 10 years is recommended in intermediate and high risk patients with contraindications or intolerance to aromatase inhibitors.
Strong recommendation, high quality level of evidence, Meta-analysis (33).
• Aromatase inhibitors should be part of the adjuvant hormonal treatment of post-menopausal women using one of the following strategies: a) Upfront use for 5 years in high risk patients and those with contraindications to tamoxifen; b) Sequential treatment with 2-3 years of AI after 2 to 3 years of tamoxifen; and c) Extended adjuvant, with 2-5 years of AI after ending first 5 years of adjuvant endocrine therapy should be discussed with all patients with intermediate and high risk disease.
Strong recommendation, high quality level of evidence, Systemic Review. (33,34)
Adjuvant Chemotherapy:
(Adjuvant chemotherapy in Triple negative, High risk Luminal B, HER2-negative):
• Adjuvant chemotherapy is recommended in all patients with triple negative, Her-2 positive, high-risk luminal HER2-negative and node positive tumors irrespective of HR status.
Strong recommendation, high quality level of evidence (35).
• We recommend a Sequential regimen of 4 cycles of anthracyclines then 4 cycles of taxane.
Strong recommendation, high quality level of evidence (35).
(Adjuvant setting in Her-2 positive, High risk Luminal B HER2-postive):
Strong recommendation, high quality level of evidence, Meta-analysis.(39, 40, 41).
• We recommend, in low risk patients (node negative tumors less than 30mm in max dimension), adjuvant trastuzumab every 3 weeks for up to 6 months (9 cycles) in combination with taxane based chemotherapy, adjuvant radiotherapy or endocrine therapy.
Conditional Moderate Quality level of evidence, Randomized Phase III ,Non-inferiority Trial. (42)
• We recommend, in Intermediate risk patients (T2-3 or N1 disease) adjuvant trastuzumab every 3 weeks for one year (17 cycles) in combination with taxane based chemotherapy, adjuvant radiotherapy or endocrine therapy.
Strong recommendation, high quality level of evidence, meta-analysis (40, 41).
• In very high risk patients (T4 or N2-3), adjuvant trastuzumab +/- pertuzumab every 3 weeks is recommended for up to one year (17 cycles) in combination with taxane based chemotherapy, adjuvant radiotherapy or endocrine therapy.
Conditional Moderate Quality level of evidence, Randomized Trial. (43)
• We recommend the TCH regimen, either combined by pertuzumab or not according to risk stratification, as a preferred systemic regimen in Her2 positive patients, especially for those with risk factors for cardiac toxicity.
Strong recommendation, high quality level of evidence, Randomized Trial. (58,59).
• Neoadjuvant Systemic Therapy
(All intrinsic subtypes):
• Neoadjuvant therapy should be indicated in all inoperable breast cancer (inflammatory BC, T4 or N2-3 disease) to allow operability irrespective of the biological subtype.
Strong recommendation, high quality level of evidence, Systemic Review /Prospective trial (47-49).
• Neoadjuvant therapy should also be indicated in operable patients to allow for breast conservation and in most patients with aggressive biological subtypes (as triple negative and HER2 positive tumors).
Strong recommendation, high quality level of evidence, Meta-analysis (49,50)
(HER2 positive subtype):
• We recommend adding pertuzumab + trastuzumab to taxane based neoadjuvant chemotherapy in HER2 positive patients cT2 or more or Node positive.
Conditional High-Quality level of evidence, Randomized Trial (45)
(Luminal A like subtype special situations):
• In post-menopausal patients with Luminal A like disease and significant co-morbidities, neoadjuvant endocrine therapy with at least 6 months of AIs should be considered.
Conditional Moderate Quality level of evidence, Systemic Review (53)
• We largely recommend adding carboplatin to neoadjuvant taxane based chemotherapy in stage II and III triple negative patients in view of event free survival improvement in addition to higher pCR rate.
Conditional recommendation Moderate Quality level of evidence, Randomized Trial (51, 52)
(Post neoadjuvant therapy):
• We recommend, in patients with clinical stage cT1-3, N0 who attained pathological complete response (ypT0/is, N0) after neoadjuvant chemotherapy and anti-HER2 therapy, completion of one year trastuzumab every 3 weeks for a total of 1 year (17 cycles) of anti-HER2 including neoadjuvant doses.
Strong recommendation, high quality level of evidence, Randomized Trial. (44).
• We recommend in patients with clinical stage cT4 or N1-3 who attained pathological complete response (ypT0, is N0) after neoadjuvant chemotherapy and anti-HER2 therapy, completion of one year of dual blockade with trastuzumab +/- pertuzumab every 3 weeks for a total of 1 year (17 cycles) of anti-HER2 including neo-adjuvant doses.
Strong recommendation, High Quality level of evidence, Randomized Trial. (43,45)
• We recommend, inpatients with TNBC who received neoadjuvant chemotherapy and who have a residual invasive disease at the time of surgery, offering adjuvant capecitabine for 6-8 cycles.
Strong recommendation High Quality level of evidence, Randomized Trial. (46)
6. Surveillance:
• History and clinical examination is recommended 2-4 times per year for 2 years then every 6 months from 3rd to 5th year then annually thereafter.
Strong recommendation, Moderate quality level of evidence, Systemic Review (55).
• Annual bilateral (after BCT) or contralateral sono-mammography (after mastectomy) is recommended.
Strong Recommendations, Moderate level of Evidence, Systemic Review (56).
➡️ Research Gaps
• Head-to-Head comparison for adjuvant Olaparib versus Capecitabine in TNBC received neoadjuvant treatment with residual disease.
• Pembrolizumab neoadjuvant exclusively versus neoadjuvant and adjuvant regarding survival benefit, toxicity including financial toxicity and quality of life.
Update of this guideline
• This guideline will be updated whenever there is new evidence.
- Annexes
Breast cancer staging:
➡️American Joint Committee on Cancer (AJCC) TNM Staging System 8th edition 2017: (60)
Primary Tumor (T) The T category of the primary tumor is defined by the same criteria regardless of whether it is based on clinical or pathological criteria, or both. The T category is based primarily on the size of the invasive component of the cancer. The maximum size of a tumor focus is used as an estimate of disease volume. The largest contiguous dimension of a tumor focus is used, and small satellite foci of noncontiguous tumor are not added to the size. The cellular fibrous reaction to invasive tumor cells is generally included in the measurement of a tumor prior to treatment; however, the dense fibrosis observed following neoadjuvant treatment is generally not included in the pathological measurement because its extent may overestimate the residual tumor volume. The clinical size of a primary tumor (T) can be measured based on clinical findings (physical examination and imaging modalities, such as mammography, ultrasound, and MR imaging) and pathological findings (gross and microscopic measurements). Clinical tumor size (cT) should be based on the clinical findings that are judged to be most accurate for a particular case, although it may still be somewhat inaccurate because the intent of some breast cancers is not always apparent with current imaging techniques and because tumors are composed of varying proportions of noninvasive and invasive disease, which these techniques are currently unable to distinguish. Size should be measured to the nearest millimeter. If the tumor size is slightly less than or greater than a cutoff for a given T classification the size should be rounded to the millimeter reading that is closest to the cutoff. For example, a reported size of 4.9 mm is reported as 5 mm, or a size of 2.04 cm is reported as 2.0 cm (20 mm). The exception to this rounding rule is for a breast tumor sized between 1.0 and 1.4 mm. These sizes are rounded up to 2 mm, because rounding down would result in the cancer’s being categorized as microinvasive carcinoma (T1mi) defined as a size of 1.0 mm or less.
➡️Definitions for T, N, M
TX Primary tumor cannot be assessed.
T0 No evidence of primary tumor
Tis (DCIS)* Ductal carcinoma in situ Tis (Paget) Paget disease of the nipple NOT associated with invasive carcinoma and/or carcinoma in situ (DCIS) in the underlying breast parenchyma. Carcinomas in the breast parenchyma associated with Paget disease are categorized based on the size and characteristics of the parenchymal disease, although the presence of Paget disease should still be noted.
T1 Tumor ≤20 mm in greatest dimension
T1mi Tumor ≤1 mm in greatest dimension
T1a Tumor >1 mm but ≤5 mm in greatest dimension (round any measurement >1.0–1.9 mm to 2 mm) T1b Tumor >5 mm but ≤10 mm in greatest dimension
T1c Tumor >10 mm but ≤20 mm in greatest dimension
T2 Tumor >20 mm but ≤50 mm in greatest dimension
T3 Tumor >50 mm in greatest dimension
T4 Tumor of any size with direct extension to the chest wall and/ or to the skin (ulceration or macroscopic nodules); invasion of the dermis alone does not qualify as T4
T4a Extension to the chest wall; invasion or adherence to pectoralis muscle in the absence of invasion of chest wall structures does not qualify as T4
T4b Ulceration and/or ipsilateral macroscopic satellite nodules and/or edema (including peau d’orange) of the skin that does not meet the criteria for inflammatory carcinoma
T4c Both T4a and T4b are present
T4d Inflammatory carcinoma
*Note: Lobular carcinoma in situ (LCIS) is a benign entity and is removed from TNM staging in the AJCC Cancer Staging Manual, 8th Edition
➡️Regional Lymph Nodes (N) Clinical (cN)
cNX* Regional lymph nodes cannot be assessed (e.g., previously removed)
cN0 No regional lymph node metastases (by imaging or clinical examination)
cN1 Metastases to movable ipsilateral level I, II axillary lymph node(s)
cN1mi** Micro metastases (approximately 200 cells, larger than 0.2 mm, but none larger than 2.0 mm)
cN2 Metastases in ipsilateral level I, II axillary lymph nodes that are clinically fixed or matted: or in ipsilateral internal mammary nodes in the absence of axillary lymph node metastases.
cN2a Metastases in ipsilateral level I, II axillary lymph nodes fixed to one another (matted) or to other structures.
cN2b Metastases only in ipsilateral internal mammary nodes in the absence of axillary lymph node metastases
cN3 Metastases in ipsilateral infraclavicular (level III axillary) lymph node(s) with or without level I, II axillary lymph node involvement; or in ipsilateral internal mammary lymph node(s) with level I, II axillary lymph node metastases; or metastases in ipsilateral supraclavicular lymph node(s) with or without axillary or internal mammary lymph node involvement
cN3a Metastases in ipsilateral infraclavicular lymph node(s)
cN3b Metastases in ipsilateral internal mammary lymph node(s) and axillary lymph node(s)
cN3c Metastases in ipsilateral supraclavicular lymph node(s)
Note: (sn) and (f) suffixes should be added to the N category to denote confirmation of metastasis by sentinel node biopsy or fine needle aspiration/core needle biopsy respectively.
*The cNX category is used sparingly in cases where regional lymph nodes have previously been surgically removed or where there is no documentation of physical examination of the axilla.
**cN1mi is rarely used but may be appropriate in cases where sentinel node biopsy is performed before tumor resection, most likely to occur in cases treated with neoadjuvant therapy.
➡️Pathologic (pN)
pNX regional lymph nodes cannot be assessed (e.g., not removed for pathological study or previously removed)
pN0 No regional lymph node metastasis identified or ITCs only
pN0(i+) ITCs only (malignant cells clusters no larger than 0.2 mm) in regional lymph node(s)
pN0(mol+) Positive molecular findings by reverse transcriptase polymerase chain reaction (RT-PCR); no ITCs detected.
pN1 Micrometastases; or metastases in 1–3 axillary lymph nodes; and/or in clinically negative internal mammary nodes with micrometastases or macrometastases by sentinel lymph node biopsy
pN1mi Micrometastases (approximately 200 cells, larger than 0.2 mm, but none larger than 2.0 mm)
pN1a Metastases in 1–3 axillary lymph nodes, at least one metastasis larger than 2.0 mm
pN1b Metastases in ipsilateral internal mammary sentinel nodes, excluding ITCs pN1c pN1a and pN1b combined.
pN2 Metastases in 4–9 axillary lymph nodes; or positive ipsilateral internal mammary lymph nodes by imaging in the absence of axillary lymph node metastases
pN2a Metastases in 4–9 axillary lymph nodes (at least one tumor deposit larger than 2.0 mm)
pN2b Metastases in clinically detected internal mammary lymph nodes with or without microscopic confirmation; with pathologically negative axillary nodes
pN3 Metastases in 10 or more axillary lymph nodes; or in infraclavicular (level III axillary) lymph nodes; or positive ipsilateral internal mammary lymph nodes by imaging in the presence of one or more positive level I, II axillary lymph nodes; or in more than three axillary lymph nodes and micro metastases or macro metastases by sentinel lymph node biopsy in clinically negative ipsilateral internal mammary lymph nodes; or in ipsilateral supraclavicular lymph nodes
pN3a Metastases in 10 or more axillary lymph nodes (at least one tumor deposit larger than 2.0 mm); or metastases to the infraclavicular (level III axillary lymph) nodes
pN3b pN1a or pN2a in the presence of cN2b (positive internal mammary nodes by imaging); or pN2a in the presence of pN1b
pN3c Metastases in ipsilateral supraclavicular lymph nodes
Note: (sn) and (f) suffixes should be added to the N category to denote confirmation of metastasis by sentinel node biopsy or FNA/core needle biopsy respectively, with NO further resection of nodes
➡️Distant Metastasis (M)
M0 No clinical or radiographic evidence of distant metastases*
cM0(i+) No clinical or radiographic evidence of distant metastases in the presence of tumor cells or deposits no larger than 0.2 mm detected microscopically or by molecular techniques in circulating blood, bone marrow, or other nonregional nodal tissue in a patient without symptoms or signs of metastases
cM1 Distant metastases detected by clinical and radiographic means
pM1 Any histologically proven metastases in distant organs; or if in non-regional nodes, metastases greater than 0.2 mm
AJCC Anatomic Stage Groups
Stage 0 Tis N0 M0 Stage IA T1 N0 M0 Stage IB T0 N1mi M0 T1 N1mi M0 Stage IIA T0 N1 M0 T1 N1 M0 T2 N0 M0 Stage IIB T2 N1 M0 T3 N0 M0 Stage IIIA T0 N2 M0 T2 N2 M0 T1 N2 M0 T3 N1 M0 T3 N2 M0 Stage IIIB T4 N0 M0 T4 N1 M0 T4 N2 M0 Stage IIIC Any T N3 M0
Stage IV Any T Any N
M1
- References
2- Perry N, Broeders M, De Wolf C, Törnberg S, Holland R, Von Karsa L. European Commission: European guidelines for quality assurance in breast cancer screening and diagnosis. Luxembourg: Office of Official Publications of the European Communities. 2006.
3- Ditsch N, Kolberg-Liedtke C, Friedrich M, et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer: Update 2021. Breast Care (Basel). 2021;16(3):214-227.
4- Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology.2008;246(1):116-124. doi:10.1148/radiol.2461061298
5- Brierley JD, Gospodarowicz MK, Wittekind C. Union for International Cancer Control. TNM Classification of Malignant Tumours. 8th ed. John Wiley & Sons, Inc.;2016
6- Gennari A, André F, Barrios CH, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475-1495
7- Gerber B, Seitz E, Müller H, et al. Perioperative screening for metastatic disease is not indicated in patients with primary breast cancer and no clinical signs of tumor spread. Breast Cancer Res Treat. 2003;82(1):29-37.
8- International Agency for Research on Cancer. Breast Tumours, WHO Classification of Tumours. 5th ed. vol 9. IARC Press; 2019
9- Wang M, He X, Chang Y, Sun G, Thabane L. A sensitivity and specificity comparison of fine needle aspiration cytology and core needle biopsy in evaluation of suspicious breast lesions: A systematic review and metaanalysis. Breast. 2017;31:157-166. doi:10.1016/j.breast.2016.11.009
10- Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch Pathol Lab Med. 2020;144(5):545-563.
11- Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol.2018;36(20):2105-2122.
12- Nielsen TO, Leung SCY, Rimm DL, et al. Assessment of Ki67 in Breast Cancer: Updated Recomendmations From the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. 2021;113(7):808-819
13- Senkus E, Gomez H, Dirix L, et al. Attitudes of young patients with breast cancer toward fertility loss related to adjuvant systemic therapies. EORTC study 10002 BIG 3-98. Psychooncology. 2014;23(2):173-182
14- Morrow M, Van Zee KJ, Solin LJ, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation in Ductal Carcinoma In Situ.J Clin Oncol. 2016;34(33):4040-4046.
15- Forbes JF, Sestak I, Howell A, et al. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): a double-blind, randomised controlled trial. Lancet.2016;387(10021):866-873.
16- Margolese RG, Cecchini RS, Julian TB, et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in Situ undergoing lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet. 016;387(10021):849-856.
17- Staley H, McCallum I, Bruce J. Postoperative tamoxifen for ductal carcinoma in situ. Cochrane Database Syst Rev. 2012;10:CD007847.
18- Josfeld L, Keinki C, Pammer C, et al. Cancer patients’ perspective on shared decision-making and decision aids in oncology. J Cancer Res Clin Oncol. 2021;147(6):1725-1732.
19- Wu ZY, Han J, Kim HJ, et al. Breast cancer outcomes following immediate breast reconstruction with implants versus autologous flaps: a propensity score-matched study. Breast Cancer Res Treat. 2022;191(2):365-373.
20- Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in Clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927-933.
21- Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318(10):918-926.
22- Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micro Metastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol.2013;14(4):297-305.
23- Moo TA, Edelweiss M, Hajiyeva S, et al. Is low-volume disease in the sentinel node after neoadjuvant chemotherapy an indication For axillary dissection? Ann Surg Oncol. 2018;25(6):1488-1494.
24- Savolt A, Peley G, Polgar C, et al. Eight-year follow up result of the OTOASOR trial: the Optimal Treatment Of the Axilla - Surgery or Radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: a randomized, single centre, phase III, non-Inferiority trial. Eur J Surg Oncol. 2017;43(4):672-679.
25- Goyal A, Mann GB, Fallowfield L, et al. POSNOC-POsitive Sentinel NOde: adjuvant therapy alone versus adjuvant therapy plus Clearance or axillary radiotherapy: a randomised controlled trial of axillary treatment in women with early-stage breast cancer who have metastases in one or two sentinel nodes. BMJ Open. 2021;11(12):e054365.
26- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S, McGale P, Correa C, et al. Effect of radiotherapy after Breast conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707-1716.
27- Murray Brunt A, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST- Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020; 395(10237):1613-1626.
28- Brunt AM, Haviland JS, Sydenham M, et al. Ten-year results of FAST: a randomized controlled trial of 5-fraction whole-breast Radiotherapy for early breast cancer. J Clin Oncol. 2020;38(28):3261-3272.
29- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), McGale P,Taylor C, Cutter D, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127-2135.
30- LHRH-agonists in Early Breast Cancer Overview group, Cuzick J, Ambroisine L, et al. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet. 2007;369(9574):1711-1723. doi:10.1016/S0140- 6736(07)60778-8
31- Francis PA, Fleming GF, Láng I, et al. Adjuvant Endocrine Therapy in Premenopausal Breast Cancer: 12-Year Results From SOFT [published correction appears in J Clin Oncol. 2023 Sep 1;41(25):4187]. J Clin Oncol. 2023;41(7):1370-1375. doi:10.1200/JCO.22.01065
32- Pagani O, Walley BA, Fleming GF, et al. Adjuvant Exemestane With Ovarian Suppression in Premenopausal Breast Cancer: Long-Term Follow-Up of the Combined TEXT and SOFT Trials. J Clin Oncol. 2023;41(7):1376-1382. doi:10.1200/JCO.22.01064
33- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level metaanalysis of the randomised trials. Lancet. 2015;386(10001):1341-1352. doi:10.1016/S0140-6736(15)61074-1
34- Gnant M, Fitzal F, Rinnerthaler G, et al. Duration of Adjuvant Aromatase Inhibitor Therapy in Postmenopausal Breast Cancer. N Engl J Med. 2021;385(5):395-405. doi:10.1056/NEJMoa2104162
35- Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432-444. doi:10.1016/S0140-6736(11)61625-5
36- Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N Engl J Med 2021;384(25):2394-2405.
37- Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376(22):2147-2159. doi:10.1056/NEJMoa1612645
38- Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30(4):558-566. doi:10.1093/annonc/mdz012.
39- Early Breast Cancer Trialists’ Collaborative group (EBCTCG). Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 2021;22(8):1139-1150. doi:10.1016/S1470-2045(21)00288-6
40- Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744-3752. doi:10.1200/JCO.2014.55.5730
41- Chumsri S, Li Z, Serie DJ, et al. Incidence of Late Relapses in Patients With HER2-Positive Breast Cancer Receiving Adjuvant Trastuzumab: Combined Analysis of NCCTG N9831 (Alliance) and NRG Oncology/NSABP B-31. J Clin Oncol. 2019;37(35):3425-3435. doi:10.1200/JCO.19.00443
42- Earl HM, Hiller L, Vallier AL, et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019;393(10191):2599-2612. doi:10.1016/S0140-6736(19)30650-6
43- Piccart M, Procter M, Fumagalli D, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer in the APHINITY Trial: 6 Years' Follow-Up. J Clin Oncol. 2021;39(13):1448-1457. doi:10.1200/JCO.20.01204
44- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and longterm clinical benefit in breast cancer: the CTNeoBC pooled analysis [published correction appears in Lancet. 2019 Mar 9;393(10175):986]. Lancet. 2014;384(9938):164-172. doi:10.1016/S0140-6736(13)62422-8
45- Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791-800. doi:10.1016/S1470-2045(16)00163-7.
46- Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147-2159.
47- Gralow JR, Burstein HJ, Wood W, et al. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008;26(5):814-819. doi:10.1200/JCO.2007.15.3510
48- Mamtani A, Barrio AV, King TA, et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients With Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Ann Surg ,Oncol. 2016;23(11):3467-3474. doi:10.1245/s10434-016-5246-8
49- Woeste MR, Bhutiani N, Donaldson M, McMasters KM, Ajkay N. Evaluating the effect of neoadjuvant chemotherapy on surgical outcomes after breast conserving surgery. J Surg Oncol. 2021;123(2):439-445. doi:10.1002/jso.26301
50- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27-39. doi:10.1016/S1470-2045(17)30777-5
51- Shepherd JH, Ballman K, Polley MC, et al. CALGB 40603 (Alliance): LongTerm Outcomes and Genomic Correlates of Response and Survival After Neoadjuvant Chemotherapy With or Without Carboplatin and Bevacizumab in Triple-Negative Breast Cancer. J Clin Oncol. 2022;40(12):1323-1334. doi:10.1200/JCO.21.01506
52- Geyer CE, Sikov WM, Huober J, et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann Oncol. 2022;33(4):384-394. doi:10.1016/j.annonc.2022.01.009
53- Spring LM, Gupta A, Reynolds KL, et al. Neoadjuvant Endocrine Therapy for Estrogen Receptor-Positive Breast Cancer: A Systematic Review and Metaanalysis. JAMA Oncol. 2016;2(11):1477-1486. doi:10.1001/jamaoncol.2016.1897
54- Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020;382(9):810-821. doi:10.1056/NEJMoa1910549
55- Montgomery DA, Krupa K, Cooke TG. Alternative methods of follow up in breast cancer: a systematic review of the literature. Br J Cancer. 2007;96(11):1625-1632. doi:10.1038/sj.bjc.6603771
56- Schootman M, Jeffe DB, Lian M, Aft R, Gillanders WE. Surveillance mammography and the risk of death among elderly breast cancer patients. Breast Cancer Res Treat. 2008;111(3):489-496. doi:10.1007/s10549-0079795-1
57- Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 2015;372:134-141. Available at: https://www.ncbi.nlm.nih.gov/pubmed/25564897.5).
58- Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracyclinecontaining and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278-2284. Available at: +http://www.ncbi.nlm.nih.gov/pubmed/23704196.
59- Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273-1283. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21991949.
60- Amin MB, Greene FL, Edge SB, Compton CC, Greenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017 Mar;67(2):93-99. Doi: 10.3322/caac.21388. Epub 2017 Jan 17. PMID: 28094848.
61- Babajan Banaganapalli Ibrahim Fallatah, Fai Alsubhi , Preetha Jayasheela Shetty , Zuhier Awan , Ramu Elango,and Noor Ahmad Shaik: Paget’s disease: a review of the epidemiology, etiology, genetics, and treatment, Front Genet. 2023; 14: 1131182.