Early & Late Laryngeal Cancer (ECPG)
| Site: | EHC | Egyptian Health Council |
| Course: | Otorhinolaryngology, Audiovestibular & Phoniatrics Guidelines |
| Book: | Early & Late Laryngeal Cancer (ECPG) |
| Printed by: | Guest user |
| Date: | Monday, 23 December 2024, 9:35 PM |
Description
"last update: 10 June 2024"
- Acknowledgements
Chief Editor: Reda Kamel1
General Secretary: Ahmed Ragab2
General Coordinator: Baliegh Hamdy3
Scientific Board: Ashraf Khaled,4 Mohamed Ghonaim,5 Mahmoud Abdelaziz,6 Tarek Ghannoum,7 Mahmoud Youssef8
Head and Neck Chief Manager: Mahmoud Abdelaziz6
Assembly Board: Mahmoud Abdelaziz,6 Eslam Farid Abu Shady,9 Mahmoud El Bestar,1 Ahmad El Naggar,6 Mohamed El Rubaie,6 Yaser Abdel Wahab Khalil,2 Abdelwahab Mohamed5
Grading Board (In alphabetical order)
Ahmad Abdel Fattah,5 Ahmad Aboul Wafa,10 Ahmad Salama Abdelmeguid,5 Hazem M. Abdeltawab,1 Sherif Askar,11 Ahmad M. Elbatawi,1 Ahmed Ali Eldegwi,16 Ahmed S. Elhamshary,6 Mohamed Eltabbakh,12 Abdel Rahman Eltahhan,12 Ahmad Eltelety,1 Khaled Gamal,13 Mahmoud Hagras,1 Ibrahim Khaled,11 Elshaarawy Mousa,5 Mohammed Roushdy,10 Abdelraof Said,11 Emad Shehata,6 Mohamed Zahran14
Reviewing Board: Magid Elshennawy,1 Mohamed Elsharnouby,15 Hisham Abdel Fattah,14 Zakria Soliman15
Other related specialties Reviewing Board: Esraa Farid Toulan17, Fatma Gharib Khirallah18, Engy Adel Asker18.
1Otorhinolaryngology Department, Faculty of Medicine/ Cairo University, 2Otorhinolaryngology Department, Faculty of Medicine/ Menoufia University, 3Otorhinolaryngology Department, Faculty of Medicine/Minia University, 4Otorhinolaryngology Department, Faculty of Medicine/ Beni-Suef University, 5Otorhinolaryngology Department, Faculty of Medicine/ Mansoura University, 6Otorhinolaryngology, H&N Department, Faculty of Medicine/Tanta University, 7Audiovestibular Unit, Otorhinolaryngology Department, Faculty of Medicine/ Cairo University,, 8 Phoniatrics Unit, Otorhinolaryngology Department, Faculty of Medicine/Ain Shams University, 9Otorhinolaryngology, Department, Faculty of Medicine/ Banha University, 10Otorhinolaryngology Department, Faculty of Medicine/ Assuit University, 11Otorhinolaryngology Department, Faculty of Medicine/ Zagazig University, 12Otorhinolaryngology Department, Faculty of Medicine/ Suez Canal University, 13Otorhinolaryngology Department, Faculty of Medicine/ Sohag University, 14Otorhinolaryngology Department, Faculty of Medicine/ Alxandria University, 15Otorhinolaryngology Department, Faculty of Medicine/ Menoufia University, 15Otorhinolaryngology Department, Faculty of Medicine/ Military Medical Academy, 16Otorhinolaryngology Department, Faculty of Medicine/ Al Azhar University.17 Phoniatrics and speech specialist at El Menshawy general hospital,Tanta , 18 Clinical oncology and radiotherapy specialist at Tanta University Hospitals, 19 Head nurse at Tanta University Hospitals.
Sincere thanks extend to the secretaries: Samar Hussein and Eman Ragab, as well as the editor: Mohamed Salah- Abbreviations
CIS Carcinoma in situ
RT Radiotherapy
VPL Vertical partial laryngectomy
CPG Clinical Practice Guideline
GRADE Grading of Recommendations Assessment, Development and Evaluation
NCCN National Comprehensive Cancer Network
MRI Magnetic Resonance Imaging
- Executive Summary
|
The purpose of this guideline is to identify quality improvement opportunities in the assessment, diagnosis, and management of laryngeal cancer and to create explicit and actionable recommendations to implement these opportunities in clinical practice.
B. Adjuvant treatment depends on the presence (or absence) of adverse features. Adjuvant treatment for selected patients with T1-2, and N0 supraglottic cancer may include re-resection if there are positive margins. For selected patients with T1-3, N+ supraglottic disease, re-resection may be attempted if negative margins are feasible and can be achieved without total laryngectomy, and if re-resection has the potential to change the indication for adjuvant systemic therapy/RT. (Strong Recommendation)
B. If total laryngectomy is indicated but laryngeal preservation is desired, concurrent systemic therapy/RT is recommended. When using systemic therapy/RT, high-dose cisplatin is preferred (at 100 mg/m2 on days 1, 22, and 43). Induction chemotherapy with management based on response is an option for all but T1-2, and N0 glottic cancer. (Strong Recommendation) C. Definitive RT (without systemic therapy) is an option for patients with T3, and N0-1 disease who are medically unfit or refuse systemic therapy. Surgery is reserved for managing the neck as indicated, for those patients whose disease persists after systemic therapy/RT or RT, or for those patients who develop a subsequent locoregional recurrence. (Strong Recommendation) D.Management of locally advanced, resectable glottic and supraglottic cancers (in which total laryngectomy is indicated but laryngeal preservation is desired) with concurrent cisplatin and radiation. Concurrent RT and systemic therapy (eg, cisplatin 100 mg/m2 preferred) is the recommended option for achieving laryngeal preservation with Long-term follow-up (10 years). (Strong Recommendation) E. In cases with T3 laryngeal cancer if IMRT and modern radiotherapy are available and affordable, concomitant radiotherapy with systemic therapy in the form of cisplastin can be tried as an organ preservation treatment. (Strong Recommendation) F. For patients with glottic and supraglottic T4a tumors, the recommended treatment approach is total laryngectomy with thyroidectomy and neck dissection as indicated (depending on node involvement) followed by adjuvant treatment (RT, or systemic therapy/RT may be considered). G. For patients with glottic T4a laryngeal cancer, postoperative observation is an option for highly selected patients with good-risk features (eg, indolent histopathology). For selected patients with T4a tumors who decline surgery, the NCCN Panel recommends: 1) considering concurrent chemoradiation; 2) clinical trials; or 3) induction chemotherapy with additional management based on response. (Strong Recommendation)
|
- Introduction, scope and audience
➡️ Introduction and definitions
Laryngeal carcinoma is the most common site of malignancy in the head and neck worldwide. The effects of the disease process and the treatment can have a significant impact on voice and swallow function and quality of life. Recent advances i.e., surgical, and non-surgical management options are available.
➡️Scope:
The purpose of this guideline is to identify quality improvement opportunities in the assessment, diagnosis, and management of laryngeal cancer and to create explicit and actionable recommendations to implement these opportunities in clinical practice. Specifically, the goals are to improve diagnostic accuracy for laryngeal cancer, promote and guide management, and the more judicious use of the surgical and non-surgical management options available.
➡️Target audience:
Target users are ENT clinicians and specialists and residents to be used for management of adult patients with cancer larynx.- Methods
➡️Methods of development
Stakeholder Involvement: Individuals who were involved in the development process. Included the above-mentioned Head and Neck Chief Manager, Head and Neck Executive Manager, Assembly Board, Grading Board and Reviewing Board.
Information about target population experiences was not applicable for this topic.
➡️Search Method
Pubmed, Medline, Egyptian Knowledge Bank, Medscape, WebMD, Google Scholar
➡️Keywords
Laryngeal cancer, chemotherapy, chemoradiation, laryngeal preservation.
➡️The adaptation cycle passed over: set-up phase, adaptation phase (Search and screen, assessment: currency, content, quality & /decision/selection) and finalization phase that included revision and external reviewing and Other related specialties Reviewing Board including phoniatrics & speech specialist, Clinical oncology & radiotherapy specialist and nurse.
➡️Time period searched: from January 2009 to March 2020.
➡️Results
Three guidelines were assessed by 7 experts Laryngologists and the International Consensus on laryngeal cancer 2021 had the highest scores as regards to the currency, contents and quality. It was graded GRADE by 19 expert Laryngologists and reviewed by 3 expert reviewers. To improve quality, gather feedback on draft recommendations. The external review was done through a rating scale as well as open-ended questions. (Annexes tables 1-3) (1).
➡️Setting: Primary, secondary and tertiary care centers & hospitals, and related specialties.Interpretation of strong and conditional recommendations for an intervention 10
|
Audience |
Strong recommendation |
Conditional recommendation |
|
Patients |
Most individuals in this situation would want the recommended course of action; only a small proportion would not. Formal decision aides are not likely to be needed to help individuals make decisions consistent with their values and preferences. |
Most individuals in this situation would want the suggested course of action, but many would not |
|
Clinicians |
Most individuals should receive the intervention. Adherence to the recommendation could be used as a quality criterion or performance indicator. |
Different choices will be appropriate for individual patients, who will require assistance in arriving at a management decision consistent with his or her values and preferences. Decision aides may be useful in helping individuals make decisions consistent with their values and preferences. |
|
Policymakers |
The recommendation can be adopted as policy in most situations. |
Policy-making will require substantial debate and involvement of various stakeholders. |
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to Decision frameworks (GRADE Working Group 2013)
|
Grade |
Definition |
|
High
|
We are very confident that the true effect lies close to that of the estimate of the effect. |
|
Moderate
|
We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
|
Low
|
Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. |
|
Very Low
|
We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect |
- Recommendations
The following statements and flowchart were adapted from the National Comprehensive Cancer Network (NCCN) 2018 (2), which had the highest scores as regards the currency, contents, and quality.

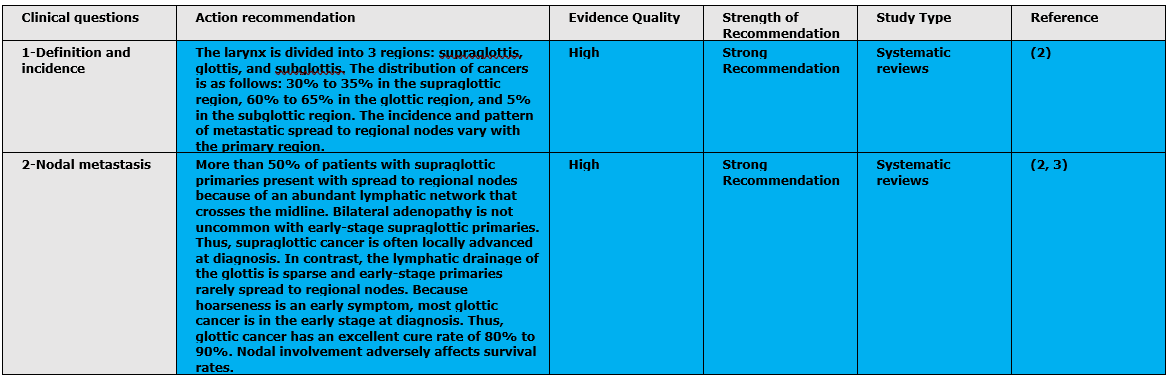
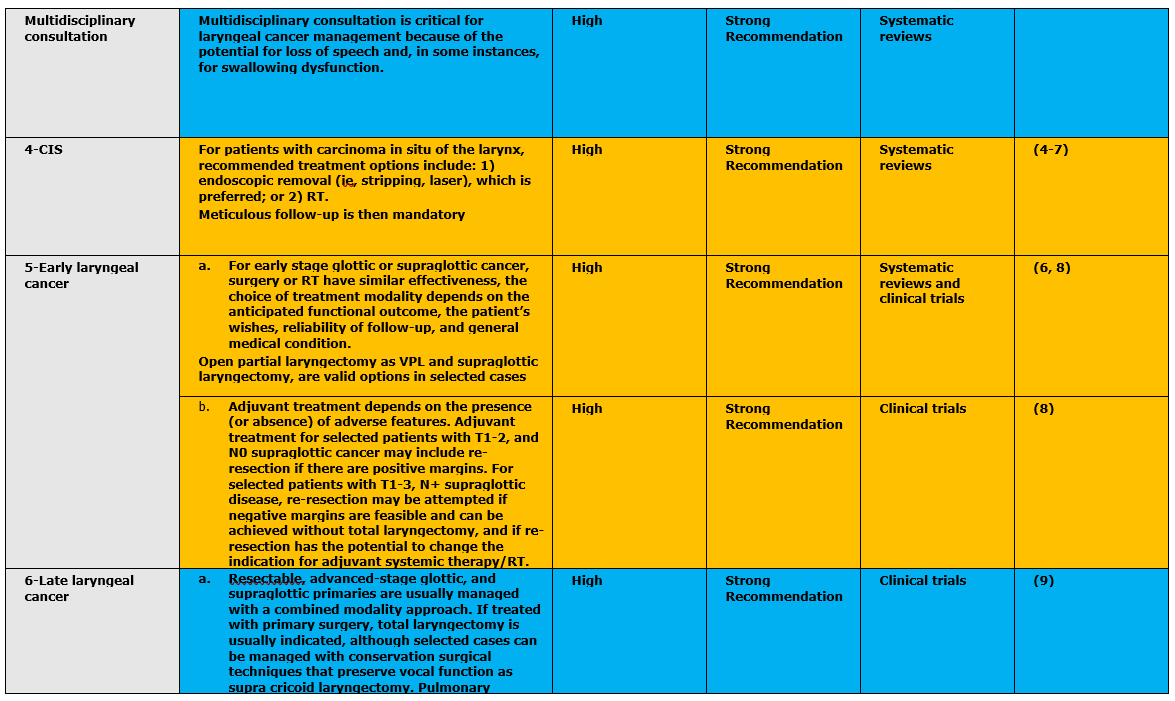


- Research needs
|
There is a need to conduct randomized controlled trials (RCTs) to further assess several problems of involvement of the anterior commissure. There is a need to conduct randomized controlled trials (RCTs) to further assess what is the appropriate treatment of the regional cervical nodes for patients with laryngeal cancer who are treated with an organ-preservation approach? There is a need to conduct randomized controlled trials (RCTs) to further assess the role of elective neck dissection in patients with radio-recurrent and radio-residual laryngeal cancer? |
- Monitoring and evaluating the impact of the guideline
Monitoring/ Auditing Criteria:
- Acquire full history from patients.
- Ensure multidisciplinary team management of cancer larynx patients.
- Perform laryngoscopic examination to all patients with cancer larynx.
- Perform direct laryngoscopic examination to all patients with cancer larynx and take biopsies for histopathological examination.
- Discuss with the patients with different management options.
- Perform regular follow up for treated patients including serial endoscopic examinations and imaging studies.
- All clinicians should be aware and informed to consider the following:
- Red Flags that need urgent referral for Assessment/ Management must be taken into consideration.
- Updating of the guideline
Updating Procedure:
Any recommendation of this guideline will be updated when new evidence that could potentially impact the current evidence base for this recommendation is identified. If no new reports or information are identified for a particular recommendation, the recommendation will be revalidated. The focus will be on recommendations supported by very-low- or low certainty evidence and where new recommendations or a change in the published recommendations may be needed.- References
1. Dijkers M. Introducing GRADE: a systematic approach to rating evidence in systematic reviews and to guideline development. KT Update. 2013;1(5):1-9.
2. Colevas AD, Yom SS, Pfister DG, Spencer S, Adelstein D, Adkins D, et al. NCCN guidelines insights: head and neck cancers, version 1.2018. Journal of the National Comprehensive Cancer Network. 2018;16(5):479-90.
3. Edge SB, American Joint Committee on Cancer ACS. AJCC cancer staging handbook: from the AJCC cancer staging manual: Springer; 2010.
4. Rödel RM, Steiner W, Müller RM, Kron M, Matthias C. Endoscopic laser surgery of early glottic cancer: involvement of the anterior commissure. Head & Neck: Journal for the Sciences and Specialties of the Head and Neck. 2009;31(5):583-92.
5. Zouhair A, Azria D, Coucke P, Matzinger O, Bron L, Moeckli R, et al. Decreased local control following radiation therapy alone in early-stage glottic carcinoma with anterior commissure extension. Strahlentherapie und Onkologie. 2004;180(2):84.
6. Warner L, Chudasama J, Kelly CG, Loughran S, McKenzie K, Wight R, et al. Radiotherapy versus open surgery versus endolaryngeal surgery (with or without laser) for early laryngeal squamous cell cancer. Cochrane Database of Systematic Reviews. 2014(12).
7. Yoo J, Lacchetti C, Hammond JA, Gilbert RW, Head, Group NCDS. Role of endolaryngeal surgery (with or without laser) versus radiotherapy in the management of early (T1) glottic cancer: a systematic review. Head & Neck. 2014;36(12):1807-19.
8. Cooper JS, Zhang Q, Pajak TF, Forastiere AA, Jacobs J, Saxman SB, et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. International Journal of Radiation Oncology* Biology* Physics. 2012;84(5):1198-205.
9. Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. Journal of clinical oncology. 2013;31(7):845.
10. Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. New England Journal of Medicine. 2003;349(22):2091-8.
- Further Reading
- Silver, C. E., Beitler, J. J., Shaha, A. R., Rinaldo, A., & Ferlito, A. (2009). Current trends in initial management of laryngeal cancer: the declining use of open surgery. European Archives of Oto-Rhino-Laryngology, 266, 1333-1352.
- Jones, T. M., De, M., Foran, B., Harrington, K., & Mortimore, S. (2016). Laryngeal cancer: United Kingdom national multidisciplinary guidelines. The Journal of Laryngology & Otology, 130(S2), S75-S82.
- Annexes
Editorial Independence:
- This guideline was developed without any external funding.
Annex 1: Guideline Flowchart
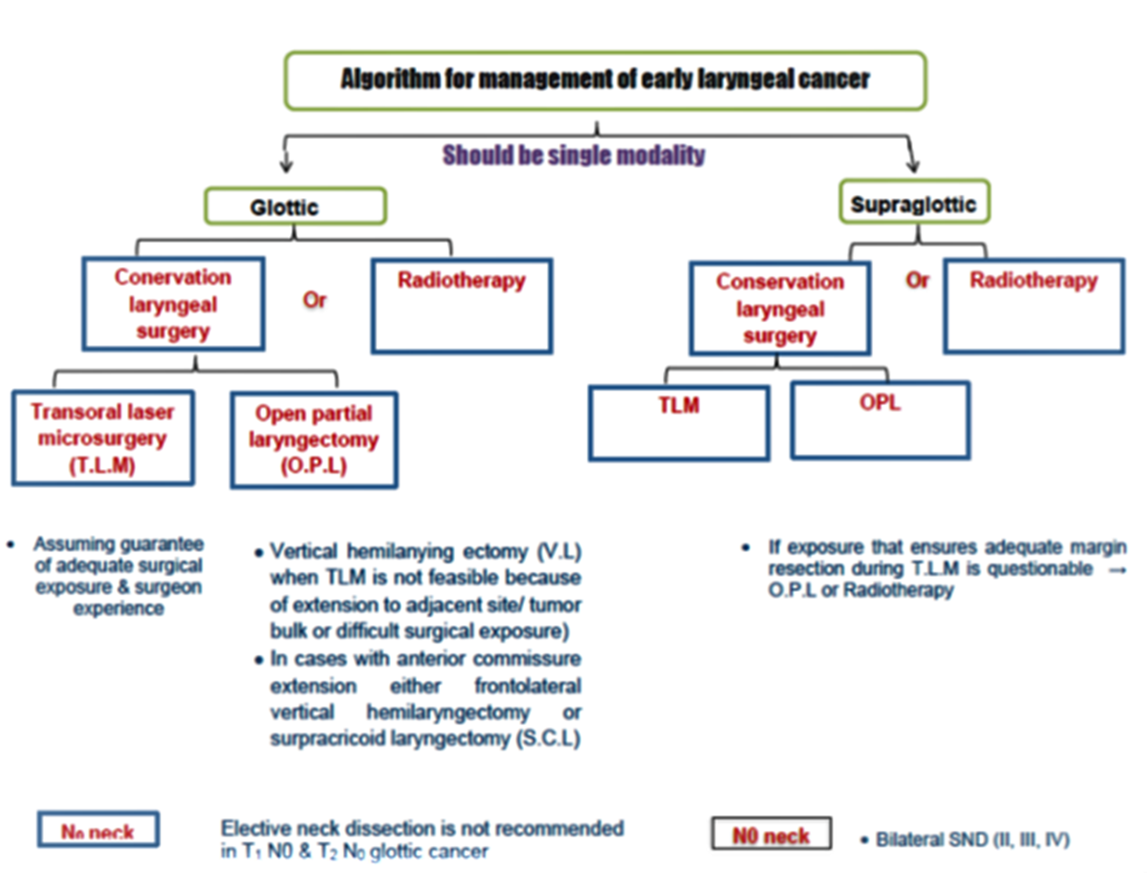
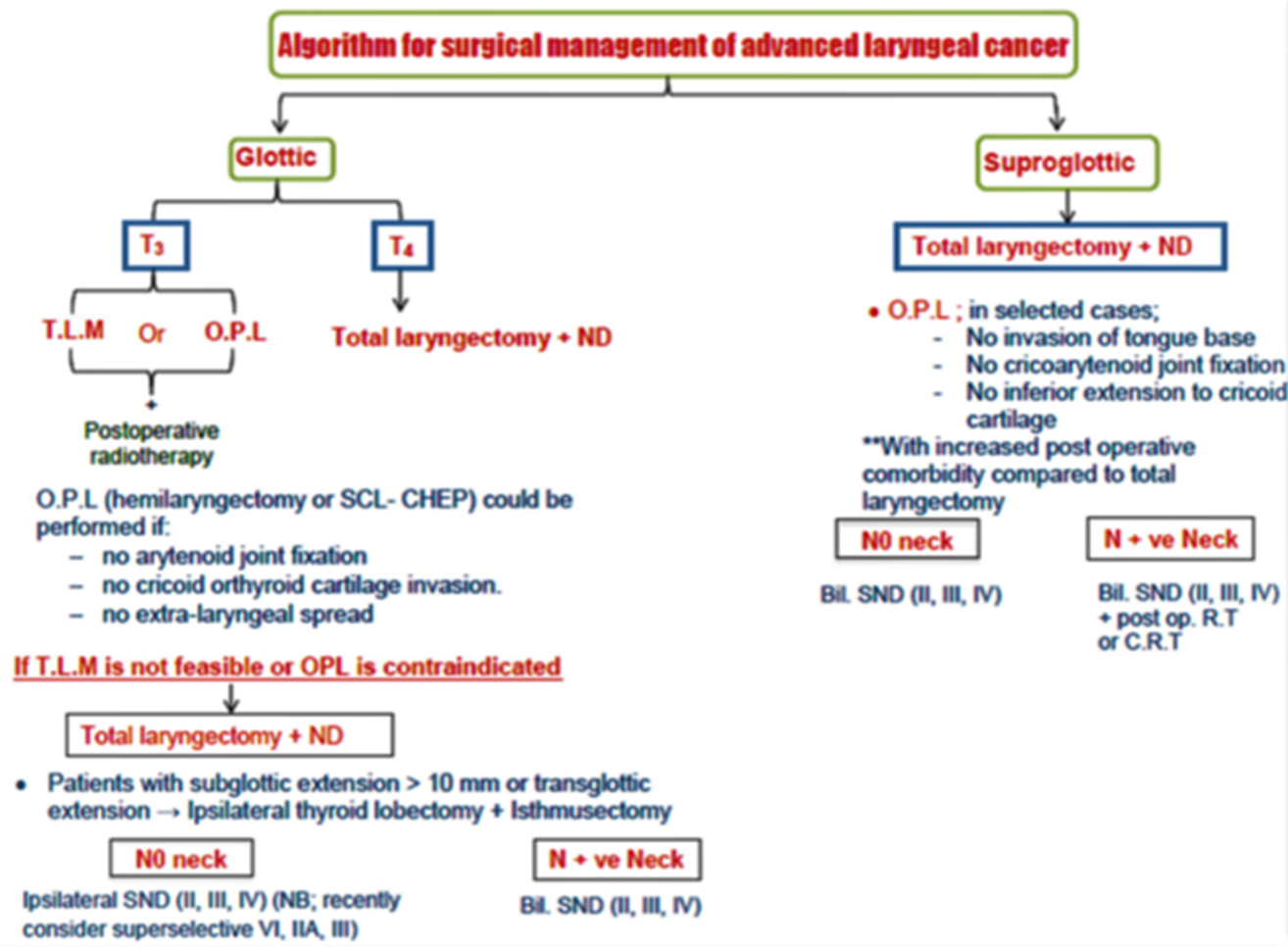
Annex 2: Tables of appraisal of selected guidelines: Currency (table 1), Content (table 2) and Quality (table 3) of the selected guidelines.
Table (1): Assessment of Currency.
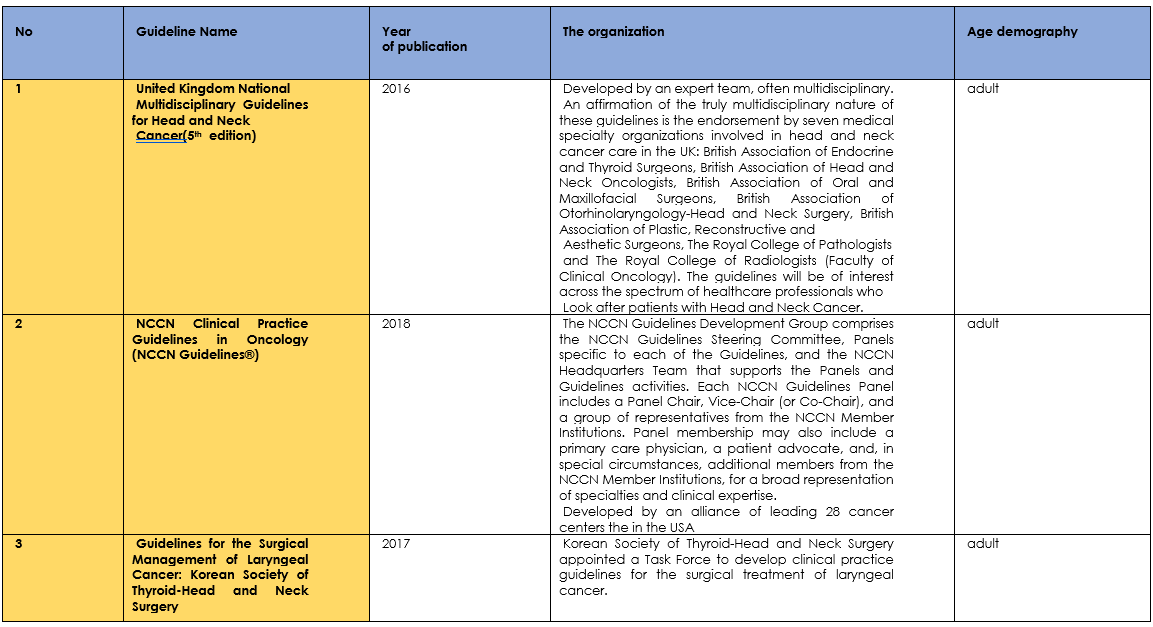
Table (2): Assessment of contents.
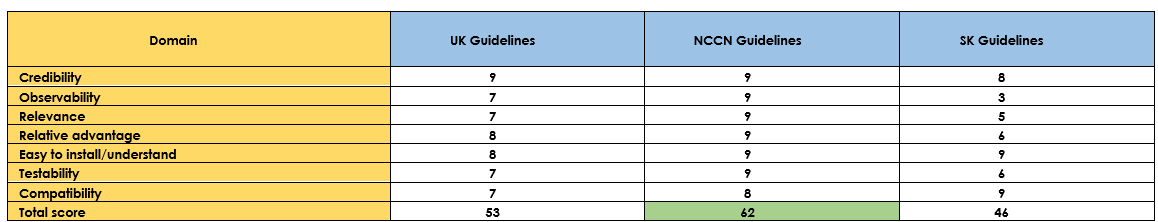
- NCCN Guidelines had the highest scores.
Table (3): Assessment of quality (CPG Appraisal tool).
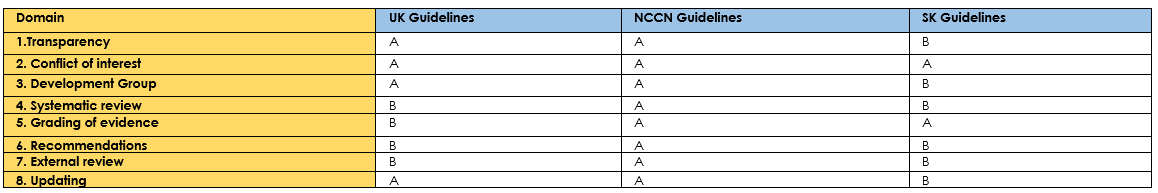
Annex 3: The risks and benefits of added and/or modified statements
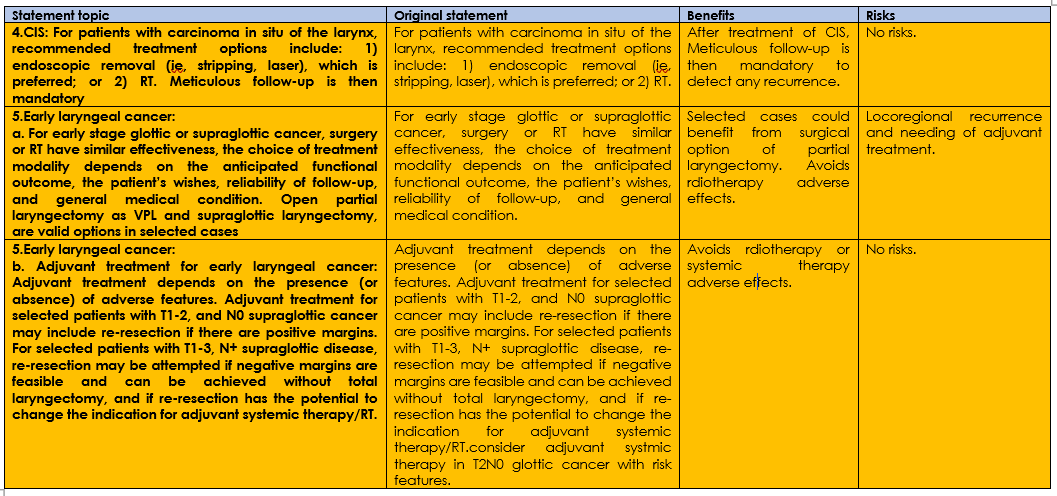
Three statements of the original guidline were modified summerized in table (4), there are no added nor omitted statements.