Critical Limb Threatening Ischemia
| Site: | EHC | Egyptian Health Council |
| Course: | Vascular Surgery Guidelines |
| Book: | Critical Limb Threatening Ischemia |
| Printed by: | Guest user |
| Date: | Monday, 23 December 2024, 10:23 PM |
Description
"last update:9 June 2024"
- Acknowledgment
We would like to acknowledge the Egyptian Health Council, the Committee of National Egyptian Guidelines, and the Vascular Scientific Committee for adapting these Guidelines.
Chair of the Panel: Mohamed Hosny, Saeed El-Mallah and Sherif Kerdawy .
Scientific Group Members: Hisham Sharaf-Eldin, Mosaad Soliman, Mohamed El-Maadawy, , Rashad Bishara, Sherif Balbaa and Ahmed El-Mahrouky.
- Abbreviations
ABI: ankle brachial index
AKA:above the knee amputation
APSV: Ankle Peak Systolic Velocity
BKA: below-the-knee amputation
CFA: common femoral artery
CLTI: Critical limb-threatening ischemia
CTA: CT angiography
DAPT:dual antiplatelet therapy
DM: Diabetes mellitus
DUS:duplex ultrasound
FP:femoropopliteal
MRA :Magnetic resonance angiography
PAD: peripheral arterial disease
PFA: profunda femoris artery
PSVR: peak systolic velocity ratio
Wifi: Wound, Ischemia, and foot Infection
- Glossary
Critical limb-threatening ischemia (CLTI) is a clinical syndrome defined by the presence of peripheral artery disease (PAD) in combination with rest pain, gangrene, or a lower limb ulceration >2 weeks duration. CLTI is associated with amputation, increased mortality, and impaired quality of life. All patients with suspected CLTI should be referred urgently to a vascular specialist.
- Scope
This Guideline is concerned with the diagnosis and treatment decisions of Critical limb-threatening ischemia (CLTI).
- Executive Summary
▪️Use ABI to determine the presence and quantify the severity of ischemia in all patients with suspected CLTI. Strong recommendation .
▪️ Perform a detailed history to determine symptoms, past medical history, and cardiovascular risk factors in all patients with suspected CLTI. Good practice statement .
▪️ Perform a complete vascular physical examination of all patients with suspected CLTI, including palpation of carotid, upper extremity, aorta, and lower extremity pulses. Good practice statement.
▪️ Perform a complete examination of both feet, including a probe-to-bone test of any open ulcers using sterile equipment, in all patients with pedal tissue loss and suspected CLTI. Good practice statement.
▪️ We recommend using DUS imaging, including assessment of Ankle Peak Systolic Velocity (APSV) as the first arterial imaging modality in patients with suspected CLTI. Strong recommendation.
▪️ We recommend other noninvasive vascular imaging modalities as CTA or MRA in patients with suspected CLTI who are candidates for revascularization, and who do not suffer impaired renal function. Strong recommendation.
▪️ Perform high-quality angiographic imaging of the lower limb using digital subtraction imaging (DSA). This should include the ankle and foot in all patients with suspected CLTI prior to proceeding to revascularization. Good practice statement.
▪️ Evaluate cardiovascular risk factors in all patients with suspected CLTI. Strong recommendation.
▪️ Refer all patients with suspected CLTI to have all modifiable risk factors including (hypertension, diabetes mellitus, and dyslipidemia), controlled to recommended levels. Strongly advise smoking cessation. Strong recommendation .
▪️ Treat all patients with CLTI with an antiplatelet agent. Strong recommendation.
▪️ We recommendclopidogrel as the single antiplatelet agent of choice in patients with CLTI. Conditional recommendation.
▪️ We recommendlow-dose aspirin and rivaroxaban, 2.5 mg twice daily, to reduce adverse cardiovascular events and lower extremity ischemic events in patients with CLTI. Conditional recommendation.
▪️ We recommend against using systemic vitamin K antagonists for the treatment of lower extremity atherosclerosis in patients with CLTI. Strong recommendation.
▪️ We recommend against using low molecular weight heparin for the treatment of lower extremity atherosclerosis in patients with CLTI, except if there is suspicion of acute thrombo-embolic event, or for bridging anticoagulation prior to an invasive procedure. Good practice statement.
▪️ We recommend the use of moderate- or high-intensity statin therapy to reduce all-cause and cardiovascular mortality in patients with CLTI. Strong recommendation.
▪️ We recommend the use of metformin as the primary hypoglycemic agent in patients with type 2 DM and CLTI. Strong recommendation.
▪️ We recommendwithholding metformin immediately before and for 24 to 48 hours after the administration of an iodinated contrast agent for diabetic patients, especially those with an estimated glomerular filtration rate <30 mL/min/1.73 m2. Conditional recommendation.
▪️ Prescribe analgesics of appropriate strength for CLTI patients who have ischemic rest pain of the lower extremity and foot until pain resolves after revascularization. Good practice statement.
▪️ Refer all patients with suspected CLTI to a vascular consultant for consideration of limb salvage unless major amputation is considered medically urgent. Good practice statement.
▪️ Offer primary amputation to patients with poor functional status (non-ambulatory), or an unsalvageable limb as judged by a qualified vascular consultant. Good practice statement .
▪️ Use an integrated threatened limb classification system (such as WIfI) to stage all CLTI patients who are candidates for limb salvage. Strong recommendation.
▪️ Perform urgent surgical drainage including minor amputation, if needed, and commence antibiotic treatment in all patients with suspected CLTI who present with deep space foot infection or wet gangrene. Perform urgent revascularization before or soon after foot surgery. Good practice statement.
▪️ Do not perform revascularization in the absence of significant ischemia (WIfI ischemia grade 0). Good practice statement.
▪️ Do not perform revascularization based on imaging alone in the absence of tissue necrosis or gangrene. Strong recommendation.
▪️ Revascularization could be performed in the absence of significant foot ischemia in exceptional conditions such as isolated region of poor perfusion, which could be the target of angiosome revascularization, if the isolated region of poor perfusion is associated with major tissue loss (eg, WIfI wound grade 2 or 3), and the wound deteriorates despite appropriate infection control, wound care, and offloading. Good practice statement.
▪️ We recommendrevascularization to all average-risk patients with moderate ischemia and extensive wounds or extensive tissue necrosis . Conditional recommendation.
▪️ Perform ultrasound vein mapping when available in all CLTI patients who are candidates for surgical bypass. Strong recommendation.
▪️ Do not classify a
CLTI patient as being unsuitable for revascularization without
review of adequate-quality imaging studies and clinical evaluation by a
qualified vascular consultant. Good practice statement.
▪️ Correct inflow disease first when both inflow and oudlow disease are present in a patient with CLTI. Good practice statement.
▪️ Base the decision for staged vs combined inflow and oudlow revascularization on patient risk and the severity of limb threat (eg, WIfI stage). Strong recommendation.
▪️ Correct inflow disease alone in CLTI patients with multilevel disease and low-grade ischemia (eg, WIfI ischemia grade 1) or limited tissue loss (eg, WIfI wound grade 0/1) and in any circumstance in which the riskbenefit of additional oudlow reconstruction is high or initially unclear. Strong recommendation.
▪️ Restage the limb and repeat the hemodynamic assessment after performing inflow correction in CLTI patients with inflow and oudlow disease. Strong recommendation.
▪️ We recommendsimultaneous inflow and oudlow revascularization in CLTI patients with a high limb risk (eg, WIfI stages 3 and 4), or in patients with severe ischemia (eg, WIfI ischemia grades 2 and 3). Conditional recommendation.
▪️ Use an endovascular-first approach for treatment of CLTI patients with moderate to severe aorto-iliac disease. Strong recommendation.
▪️ We recommendsurgical reconstruction for the treatment of average-risk CLTI patients with extensive aorto- iliac disease, or after failed endovascular intervention. Conditional recommendation.
▪️ Perform open CFA endarterectomy with patch angioplasty, with or without extension into the PFA, in CLTI patients with hemodynamically significant (>50% stenosis) disease of the common and deep femoral arteries. Strong recommendation.
▪️ We recommenda hybrid procedure combining open CFA endarterectomy and endovascular treatment of aorto-iliac disease with concomitant CFA involvement. Conditional recommendation.
▪️ We recommendendovascular treatment of significant CFA disease in selected patients who are deemed to be at high surgical risk or to have a hostile groin. Conditional recommendation.
▪️ Avoid stents in the CFA and do not place stents across the origin of a patent deep femoral artery. Good practice statement.
▪️ Correct hemodynamically significant (>50% stenosis) disease of the proximal deep femoral artery whenever technically feasible. Good practice statement.
▪️ In surgically average-risk CLTI patients with infrainguinal disease, base decisions of endovascular intervention vs open surgical bypass on the severity of limb threat (eg, WIfI), the anatomic pabern of disease, and the availability of autologous vein. Strong recommendation.
▪️ Offer endovascular revascularization when technically feasible for surgically high-risk patients with advanced limb threat and significant perfusion deficits (eg, WIfI ischemia grades 2 and 3). Conditional recommendation.
▪️ We recommendangiosome-guided revascularization in patients with significant wounds (eg, WIfI wound grades 3 and 4), particularly those involving the midfoot or hindfoot, and when the appropriate target arterial path is available. Conditional recommendation.
▪️ In treating femoropopliteal (FP) disease in CLTI patients by endovascular means, We recommendadjuncts to balloon angioplasty (eg, stents, covered stents, or drug-eluting technologies) when appropriate. Conditional recommendation.
▪️ Use autologous vein as the preferred conduit for infrainguinal bypass surgery in CLTI. Strong recommendation.
▪️ Avoid using a non-autologous conduit for infrainguinal bypass unless there is no endovascular option and no adequate autologous vein. Conditional recommendation.
▪️ We recommendperforming intraoperative imaging (angiography, DUS, or both) on completion of open bypass surgery for CLTI and correct significant technical defects if feasible during the index operation. Strong recommendation.
▪️ We recommend against usinglumbar sympathectomy for limb salvage in CLTI patients in whom revascularization is not possible, except in carefully selected cases. Conditional recommendation.
▪️ We recommendintermibent pneumatic compression therapy in carefully selected patients (eg, rest pain, minor tissue loss) in whom revascularization is not possible. Conditional recommendation.
▪️ Do not offer prostanoids for limb salvage in CLTI patients. We recommendoffering selectively for patients with rest pain or minor tissue loss and in whom revascularization is not possible. Conditional recommendation.
▪️ Do not offer vasoactive drugs in patients in whom revascularization is not possible. Strong recommendation.
▪️ Do not offer hyperbaric oxygen therapy to improve limb salvage in CLTI patients with severe, uncorrected ischemia (eg, WIfI ischemia grade 2/3). Strong recommendation.
▪️ The patient should be provided with optimal wound care until the lower extremity wound is completely healed or the patient undergoes amputation. Good practice statement.
▪️ Restrict the use of therapeutic angiogenesis to CLTI patients who are enrolled in a registered clinical trial. Strong recommendation.
▪️ We recommendtrans metatarsal amputation of the forefoot in CLTI patients who would require more than two digital ray amputations to resolve distal necrosis, especially when the hallux is involved. Conditional recommendation.
▪️ Offer primary amputation to CLTI patients who have a pre-existing dysfunctional or unsalvageable limb, or a poor functional status (eg, bedridden), after shared decision-making with the patient and health care team. Strong recommendation.
▪️ We recommendsecondary amputation for patients with CLTI who have a failed or ineffective reconstruction and in whom no further revascularization is possible and who have incapacitating pain, nonhealing wounds, or uncontrolled sepsis in the affected limb after shared decision-making with the patient and health care team. Conditional recommendation.
▪️ We recommendrevascularization to improve the possibility of healing an amputation at a more distal functional amputation level (eg, AKA to BKA), particularly for patients with a high likelihood of rehabilitation and continued ambulation. Conditional recommendation.
▪️ We recommenda BKA or AKA in patients who are non-ambulatory for reasons other than CLTI (ie, bedridden patients with flexion contracture, dense hemiplegia, cancer) and are unlikely to undergo successful rehabilitation to ambulation after shared decision-making with the patient and health care team. Conditional recommendation.
▪️ Patients who have undergone amputation for CLTI are instructed to seek medical advice to monitor the progression of disease in the contralateral limb and to maintain optimal medical therapy and risk factor management. Strong recommendation.
▪️ Postprocedural care and surveillance of infrainguinal revasculariza%on for CLTI
▪️ Continue best medical therapy for PAD, including the long-term use of antiplatelet and statin therapies, in all patients who have undergone lower extremity revascularization. Strong recommendation.
▪️ Promote smoking cessation in all CLTI patients who have undergone lower extremity revascularization. Strong recommendation.
▪️ We recommendDAPT (aspirin plus clopidogrel) in patients who have undergone infrainguinal prosthetic bypass for CLTI for a period of 6 to 24 months to maintain graft patency. Conditional recommendation.
· We recommendDAPT (aspirin plus clopidogrel) in patients who have undergone infrainguinal endovascular interventions for CLTI for a period of at least 1 month, alternatively, We recommendAspirin and Rivaroxaban 2.5mg bd. Conditional recommendation.
▪️ We recommendDAPT for a period of 1 to 6 months in patients undergoing repeated catheter-based interventions, or alternatively We recommendAspirin and Rivaroxaban 2.5mg bd, if they are at low risk for bleeding. Conditional recommendation.
▪️ Patients who have undergone lower extremity vein or prosthesis bypass for CLTI are advised to have a check-up on a regular basis for at least 2 years. We recommendDUS scanning where available. Good practice statement.
▪️ We recommendperforming additional imaging in patients with lower extremity grafts who have a decrease in ABI or a decrease in APSV or recurrence of symptoms or a change in pulse status to detect vein graft stenosis. Good practice statement.
▪️ Offer intervention for DUS-detected vein graft lesions with an associated PSV of >300 cm/s and a PSV ratio >3.5 or grafts with low velocity (mid-graft PSV <45 cm/s), which may be further documented by CTA, to maintain graft patency. Strong recommendation.
▪️ The patient is advised to maintain long-term surveillance after surgical or catheter-based revision of a vein graft, including DUS graft scanning where available, to detect recurrent graft-threatening lesions. Strong recommendation.
▪️ Refer for mechanical offloading as a primary component for care of all CLTI patients with plantar wounds, and for continued protection of the healed wound and the foot to include appropriate shoes, insoles, and monitoring of inflammation. Strong recommendation.
- Introduction
The incidence of PAD has increased over the years due to population aging and the global epidemic of diabetes. Some patients progress to CLTI , an advanced stage of PAD. CLTI is associated with increased mortality, risk of amputation, and impaired quality of life. CLTI is a clinical syndrome defined by the presence of PAD in combination with rest pain, gangrene, or a lower limb ulceration >2 weeks duration.
- Purpose
The purpose of this multidisciplinary guideline is to identify quality improvement opportunities in managing CLTI and to create explicit and actionable recommendations to implement these opportunities in clinical practice.
Specifically, the goals are to improve diagnostic accuracy, identify Patients who are most susceptible to CLTI, and educate clinicians and patients regarding the evidence based methods of diagnosis and treatment of different stages and complications of the disease.
- The target audience
The guideline is intended for all clinicians who are likely to diagnose and manage patients with CLTI, and it applies to any setting in which CLTI would be identified, monitored, or managed.
- Methods
A comprehensive search for guidelines was undertaken to identify the most relevant guidelines to We recommendfor adaptation.
inclusion/exclusion criteria followed in the search and retrieval of guidelines to be adapted:
• Selecting only evidence-based guidelines (guideline must include a report on systematic literature searches and explicit links between individual recommendations and their supporting evidence)
• Selecting only national and/or international guidelines
• Specific range of dates for publication (using Guidelines published or updated 2015 and later)
• Selecting peer reviewed publications only
• Selecting guidelines written in English language
• Excluding guidelines written by a single author not on behalf of an organization in order to be valid and comprehensive, a guideline ideally requires multidisciplinary input
• Excluding guidelines published without references as the panel needs to know whether a thorough literature review was conducted and whether current evidence was used in the preparation of the recommendations
The following characteristics of the retrieved guidelines were summarized in a table:
• Developing organization/authors
• Date of publication, posting, and release
• Country/language of publication
• Date of posting and/or release
• Dates of the search used by the source guideline developers
All retrieved Guidelines were screened and appraised using the AGREE II instrument (www.agreetrust.org) by at least two members. the panel decided on a cut-off point or ranked the guidelines (any guideline scoring above 50% on the rigor dimension was retained) . This guideline has been adapted from global vascular guidelines on the management of CLTI (2019)
- Evidence assessment
According to WHO handbook for Guidelines we used the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach to assess the quality of a body of evidence, develop and report recommendations. GRADE methods are used by WHO because these represent internationally agreed standards for making transparent recommendations. Detailed information on GRADE is available through the GRC secretariat and on the following sites:
■ GRADE working group: http://www.gradeworkingroup.org
■ GRADE online training modules: http://cebgrade.mcmaster.ca/ ■ GRADE profile software: http://ims.cochrane.org/revman/gradeproTable 1 Quality of evidence in GRADE
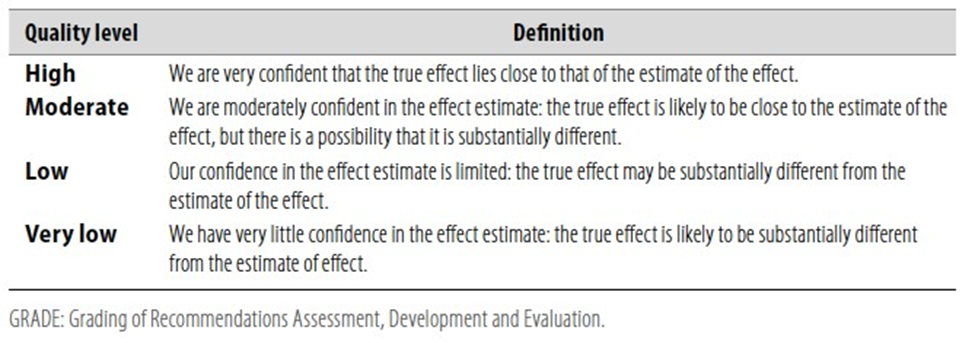 Table 2 Significance of the four levels of evidence
Table 2 Significance of the four levels of evidence
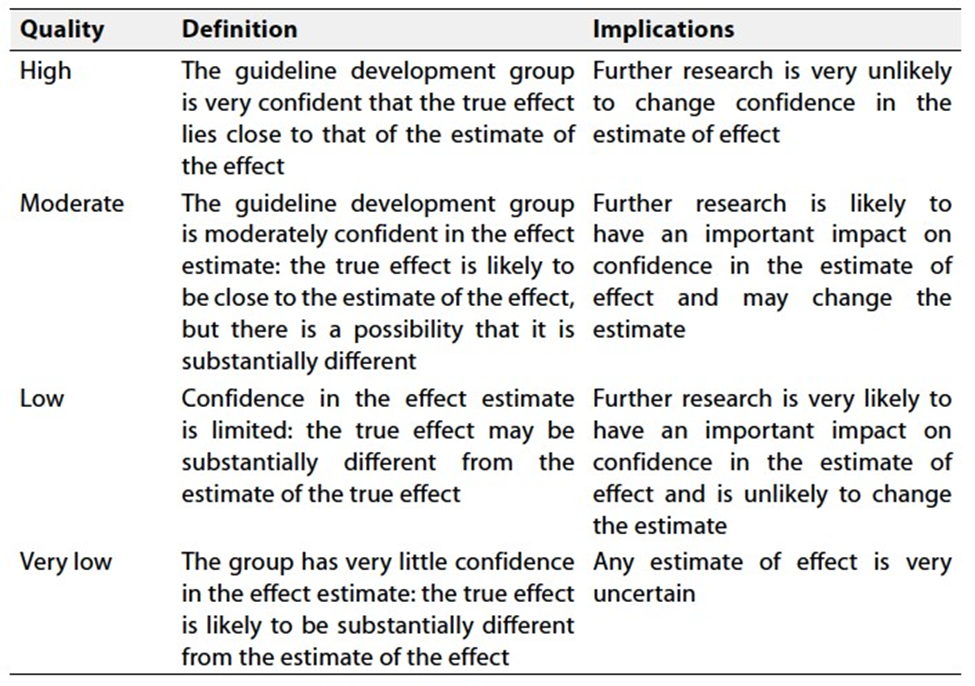
Table 3 Factors that determine How to upgrade or downgrade the quality of evidence
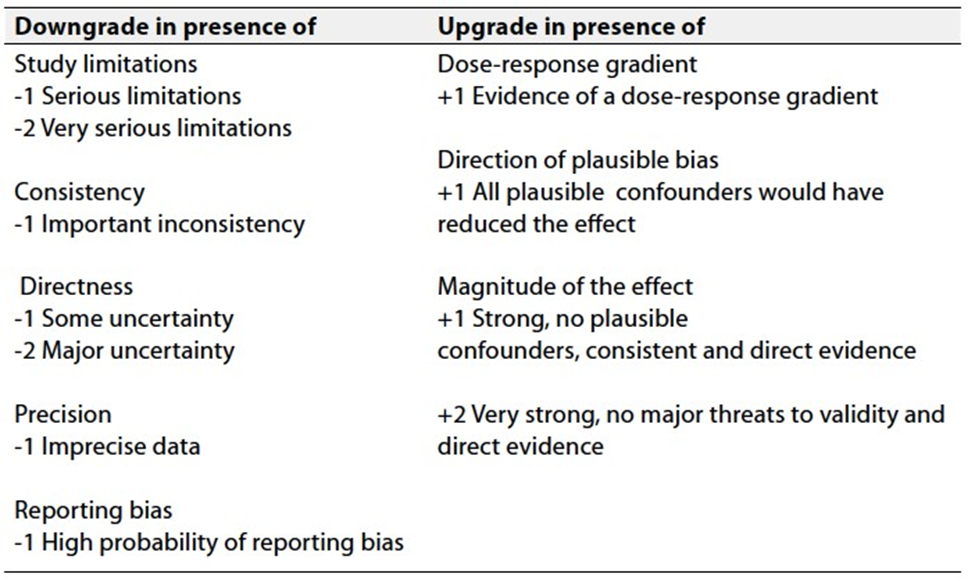
- The strength of the recommendation
The strength of a recommendation communicates the importance of adherence to the recommendation.
Strong recommendations
With strong recommendations, the guideline communicates the message that the desirable effects of adherence to the recommendation outweigh the undesirable effects. This means that in most situations the recommendation can be adopted as policy.
Conditional recommendations
These are made when there is greater uncertainty about the four factors above or if local adaptation has to account for a greater variety in values and preferences, or when resource use makes the intervention suitable for some, but not for other locations. This means that there is a need for substantial debate and involvement of stakeholders before this recommendation can be adopted as policy.
When not to make recommendations
When there is lack of evidence on the effectiveness of an intervention, it may be appropriate not to make a recommendation.
- Recommendations
|
Recommendation |
Strength of recommendation |
Quality of evidence |
References |
|
1.Diagnosis |
|
|
|
|
1.1 Use ABI to determine the presence and to quantify the severity of ischemia in all patients with suspected CLTI |
strong |
Low |
[1] [2] [3] |
|
1.2 Perform a detailed history to determine symptoms, past medical history, and cardiovascular risk factors in all patients with suspected CLTI. |
Good practice statement |
||
|
1.3 Perform a complete vascular physical examination of all patients with suspected CLTI, including palpation of carotid, upper extremity, aorta, and lower extremity pulses. |
Good practice statement |
||
|
1.4 Perform a complete examination of both feet, including a probe-to- bone test of any open ulcers using sterile equipment, in all patients with pedal tissue loss and suspected CLTI. |
Good practice statement |
||
|
1.5 We recommendDUS imaging, including assessment of Ankle Peak Systolic Velocity (APSV) as the first arterial imaging modality in patients with suspected CLTI. |
Strong |
Moderate |
[4] |
|
1.6 We recommendother noninvasive vascular imaging modalities (CTA, MRA) in patients with suspected CLTI who are candidates for revascularization, and who do not suffer impaired renal function. |
strong |
Moderate |
[5] [6] [7] [8] |
|
1.7 Obtain high-quality angiographic imaging of the lower limb using digital subtraction imaging (DSA). This should include the ankle and foot in all patients with suspected CLTI prior to proceeding to revascularization. |
Good practice statement |
|
2. Medical management |
|
|
|
|
2.1 Evaluate cardiovascular risk factors in all patients with suspected CLTI. |
strong |
Moderate |
[9] |
|
2.2 Refer all patients with suspected CLTI to have all modifiable risk factors including hypertension, diabetes mellitus, dyslipidemia, controlled to recommended levels. Strongly advice smoking cessation. |
strong |
Moderate |
[10], [11] |
|
2.3 Treat all patients with CLTI with an antiplatelet agent. |
strong |
High |
[12] |
|
2.4 We recommendclopidogrel as the single antiplatelet agent of choice in patients with CLTI. |
Conditional |
Moderate |
[13],[14] |
|
2.5 We recommendlow-dose aspirin and rivaroxaban, 2.5 mg twice daily, to reduce adverse cardiovascular events and lower extremity ischemic events in patients with CLTI. |
Conditional |
Moderate |
[15] |
|
2.6 We recommend against usingsystemic vitamin K antagonists for the treatment of lower extremity atherosclerosis in patients with CLTI. |
strong |
Moderate |
[15] |
|
2.7 We recommend against usinglow molecular weight heparin for the treatment of lower extremity atherosclerosis in patients with CLTI, except if there is suspicion of acute thrombo-embolic event, or for bridging anticoagulation prior to an invasive procedure. |
Good practice statement |
||
|
2.8 Use moderate- or high-intensity statin therapy to reduce all-cause and cardiovascular mortality in patients with CLTI. |
strong |
High |
[16] [17] [16] [18] [19] |
|
2.9 Use medormin as the |
strong |
High |
[20] |
|
2.10 We recommendwithholding medormin immediately before and for 24 to 48 hours after the administration of an iodinated contrast agent for diabetic patients, especially those with an estimated glomerular filtration rate <30 mL/min/1.73 m2. |
Conditional |
Low |
[21] [22] [23] |
|
2.11 Prescribe analgesics of appropriate strength for CLTI patients who have ischemic rest pain of the lower extremity and foot until pain resolves after revascularization. |
Good practice statement |
|
3. Evidence Based Revascularization (EBR) |
|
|
|
||
|
3.1 Refer all patients with suspected CLTI to a vascular consultant for consideration of limb salvage unless major amputation is considered medically urgent. |
Good practice statement |
||||
|
3.2 Offer primary amputation to patients with poor functional status (non-ambulatory), or an unsalvageable limb as judged by a qualified vascular consultant. |
Good practice statement |
||||
|
3.3 Use an integrated threatened limb classification system (such as WIfI) to stage all CLTI patients who are candidates for limb salvage. |
strong |
Low |
[24] [25] [26], [27], [28] |
||
|
3.4 Perform urgent surgical drainage including minor amputation, if needed, and commence antibiotic treatment in all patients with suspected CLTI who present with deep space foot infection or wet gangrene. Perform urgent revascularization before or soon after foot surgery. |
Good practice statement |
||||
|
3.5 Do not perform revascularization in the absence of significant ischemia (WIfI ischemia grade 0). |
Good practice statement |
||||
|
3.6 Do not perform revascularization based on imaging alone in the absence of tissue necrosis or gangrene. |
Good practice statement |
||||
|
3.7 Revascularization could be performed in the absence of significant foot ischemia in exceptional conditions such as isolated region of poor perfusion, which could be the target of angiosome revascularization, if the isolated region of poor perfusion is associated with major tissue loss (eg, WIfI wound grade 2 or 3), and the wound deteriorates despite appropriate infection control, wound care, and offloading. |
Good practice statement |
||||
|
3.7 Offer revascularization to all average-risk patients with severe ischemia and tissue necrosis or gangrene |
strong |
Low |
[29] |
||
|
3.8 We recommendrevascularization to all average-risk patients with moderate ischemia and extensive wounds or extensive tissue necrosis |
Conditional |
Low |
[25] [26],[27], [28] |
||
|
3.9 Perform ultrasound vein mapping in all CLTI patients who are candidates for surgical bypass. |
strong |
Low |
[30], [31], [32] |
||
|
3.10 Do not classify a CLTI patient as being unsuitable for revascularization without
|
Good practice statement |
||||
|
4.0 Combined inflow and outflow disease |
|
|
|
|
4.1 Correct inflow disease first when both inflow and outflow diseases are present in a patient with CLTI. |
Good practice statement |
||
|
4.2 Base the decision for staged vs combined inflow and oudlow revascularization on patient risk and the severity of limb threat (eg, WIfI stage). |
strong |
Low |
[30] [33] |
|
4.3 Correct inflow disease alone in CLTI patients with multilevel disease and low-grade ischemia (eg, WIfI ischemia grade 1) or limited tissue loss (eg, WIfI wound grade 0/1) and in any circumstance in which the riskbenefit of additional oudlow reconstruction is high or initially unclear. |
strong |
Low |
[30] [33] |
|
4.4 Restage the limb and repeat the hemodynamic assessment after performing inflow correction in CLTI patients with inflow and oudlow disease |
strong |
Low |
[34] |
|
4.5 We recommendsimultaneous inflow and oudlow revascularization in CLTI patients with a high limb risk (eg, WIfI stages 3 and 4), or in patients with severe ischemia (eg, WIfI ischemia grades 2 and 3). |
Conditional |
Low |
[35] |
|
5.0 Aorto-iliac disease |
|
|
|
|
5.1 Use an endovascular-first approach for treatment of CLTI patients with moderate to severe aorto-iliac disease. |
strong |
Moderate |
[36], [37], [38] |
|
5.2 We recommendsurgical reconstruction for the treatment of average-risk CLTI patients with extensive aorto- iliac disease, or after failed endovascular intervention. |
Conditional |
Low |
[39],[40],[41] |
|
6.0 Common femoral artery disease |
|
|
|
|
6.1 Perform open CFA endarterectomy with patch angioplasty, with or without extension into the PFA, in CLTI patients with hemodynamically significant (>50% stenosis) disease of the common and deep femoral arteries. |
strong |
Low |
[42],[43] |
|
6.2 We recommenda hybrid procedure combining open CFA endarterectomy and endovascular treatment of aorto-iliac disease with concomitant CFA involvement. |
Conditional |
Low |
[44] |
|
6.3 We recommendendovascular treatment of significant CFA disease in selected patients who are deemed to be at high surgical risk or to have a hostile groin. |
Conditional |
Low |
[45], [46], [47], [48] |
|
6.4 Avoid stents in the CFA and do not place stents across the origin of a patent deep femoral artery. |
Good practice statement |
|
|
|
6.5 Correct hemodynamically significant (>50% stenosis) disease of the proximal deep femoral artery whenever technically feasible. |
Good practice statement |
|
|
|
7.0 endovascular vs surgical bypass
|
|
|
|
|
|
7.1 In surgically average-risk CLTI patients with infrainguinal disease, base decisions of endovascular intervention vs open surgical bypass on the severity of limb threat (eg, WIfI), the anatomic pabern of disease, and the availability of autologous vein. |
strong |
Low |
[49] |
|
|
7.2 Offer endovascular revascularization when technically feasible for surgically high-risk patients with advanced limb threat and significant perfusion deficits (eg, WIfI ischemia grades 2 and 3). |
Conditional |
Low |
[29],[26],[27], [28] |
|
|
7.3 We recommendangiosome-guided revascularization in patients with significant wounds (eg, WIfI wound grades 3 and 4), particularly those involving the midfoot or hindfoot, and when the appropriate target arterial path is available. |
Conditional |
Low |
[50],[51],[52], [53], [54] |
|
|
7.4 In treating femoro-popliteal (FP) disease in CLTI patients by endovascular means, We recommendadjuncts to balloon angioplasty (eg, stents, covered stents, or drug- eluting technologies) when appropriate. |
Conditional |
Moderate |
[55], [56], [57], [58],[49] |
|
|
7.5 Use autologous vein as the preferred conduit for infrainguinal bypass surgery in CLTI. |
strong |
Moderate |
[49] |
|
|
7.6 Avoid using a non-autologous conduit for infrainguinal bypass unless there is no endovascular option and no adequate autologous vein. |
2(Conditional) |
C (Low) |
[49] |
|
|
7.7 We recommendperforming intraoperative imaging (angiography, DUS, or both) on completion of open bypass surgery for CLTI and correct significant technical defects if feasible during the index operation. |
strong |
Low |
[59], [60] |
|
|
8.0 Non-revascularization treatment of the limb |
|
|
|
|
8.1 We recommend against usinglumbar sympathectomy for limb salvage in CLTI patients in whom revascularization is not possible. |
Conditional |
Low |
[61] |
|
8.2 We recommendintermibent pneumatic compression therapy in carefully selected patients (eg, rest pain, minor tissue loss) in whom revascularization is not possible. |
Conditional |
Moderate |
[29] |
|
8.3 Do not offer prostanoids for limb salvage in CLTI patients. We recommendoffering selectively for patients with rest pain or minor tissue loss and in whom revascularization is not possible. |
Conditional |
Moderate |
[62] |
|
8.4 Do not offer vasoactive drugs in patients in whom revascularization is not possible. |
strong |
Low |
[63] |
|
8.5 Do not offer hyperbaric oxygen therapy to improve limb salvage in CLTI patients with severe, uncorrected ischemia (eg, WIfI ischemia grade 2/3). |
strong |
Moderate |
[64], [65], [66] |
|
8.6 The patient should be provided with optimal wound care until the lower extremity wound is completely healed or the patient undergoes amputation. |
Good practice statement |
||
|
8.7 Restrict use of therapeutic angiogenesis to CLTI patients who are enrolled in a registered clinical trial. |
strong |
Moderate |
[29], [67] |
|
9.0 The role of minor and major amputations |
|
|
|
|
9.1 We recommendtransmetatarsal amputation of the forefoot in CLTI patients who would require more than two digital ray amputations to resolve distal necrosis, especially when the hallux is involved. |
Conditional |
Low |
[68] |
|
9.2 Offer primary amputation to CLTI patients who have a pre-existing dysfunctional or unsalvageable limb, a poor functional status (eg, bedridden), after shared decision- making with the patient and health care team. |
strong |
Low |
[69],[70] |
|
9.3 We recommendsecondary amputation for patients with CLTI who have a failed or ineffective reconstruction and in whom no further revascularization is possible and who have incapacitating pain, nonhealing wounds, or uncontrolled sepsis in the affected limb after shared decision-making with the patient and health care team. |
Conditional |
Low |
[71] |
|
9.4 We recommendrevascularization to improve the possibility of healing an amputation at a more distal functional amputation level (eg, |
Conditional |
Low |
[72] |
|
9.5 We recommenda BKA or AKA in patients who are non-ambulatory for reasons other than CLTI (ie, bedridden patients with flexion contracture, dense hemiplegia, cancer) and are unlikely to undergo successful rehabilitation to ambulation after shared decision- making with the patient and health care team |
Conditional |
Low |
[73] [74] |
|
9.7 Patients who have undergone amputation for CLTI are instructed to seek medical advice to monitor progression of disease in the contralateral limb and to maintain optimal medical therapy and risk factor management. |
strong |
Low |
[75] [76] |
|
10.0 Postprocedural care and
surveillance after
infrainguinal revascularization
for CLTI |
|||
|
10.1 Continue best medical therapy for PAD, including the long-term use of antiplatelet and statin therapies, in all patients who have undergone lower extremity revascularization. |
strong |
High |
[77], [78], [79], [80], [81] |
|
10.2 We strongly advise smoking cessation in all CLTI patients who have undergone lower extremity revascularization. |
strong |
High |
[82], [83] |
|
10.3 We recommendDAPT (aspirin plus clopidogrel) in patients who have undergone infrainguinal prosthetic bypass for CLTI for a period of 6 to 24 months to maintain graft patency. |
Conditional |
Moderate |
[79], [84], [85], [80] |
|
10.4 We recommendDAPT (aspirin plus clopidogrel) in patients who have undergone infrainguinal endovascular interventions for CLTI for a period of at least 1 month, alternatively We recommendAspirin and Rivaroxiban 2.5mg bd. |
Conditional |
Low |
[86], [86], [87], [88] |
|
10.5 We recommendDAPT for a period of 1 to 6 months in patients undergoing repeated catheter based interventions, or alternatively We recommendAspirin and Rivaroxiban 2.5mg bd, if they are at low risk for bleeding. |
Conditional |
Low |
[89], [87], [88] |
|
10.6 Patients who have undergone lower extremity vein or prosthesis bypass for CLTI are advised to have a check up on a regular basis for at least 2 years. We recommendDUS scanning where available. |
Good practice statement |
||
|
10.7 We recommendperforming additional imaging in patients with lower extremity grafts who have a decrease in ABI or a decrease in APSV or recurrence of symptoms or change in pulse status to detect vein graft stenosis. |
Good practice statement |
||
|
10.8 Offer intervention for DUS- detected vein graft lesions with an associated PSV of >300 cm/s and a PSV ratio >3.5 or grafts with low velocity (mid-graft PSV <45 cm/s), which may be further documented by CTA, to maintain graft patency. |
strong |
Moderate |
[90],138 2001 |
|
10.9 Patient is strongly advised to maintain long-term surveillance after surgical or catheter-based revision of a vein graft, including DUS graft scanning where available, to detect recurrent graft-threatening lesions. |
strong |
Moderate |
[91], [92] |
|
10.10 Refer for mechanical offloading as a primary component for care of all CLTI patients with plantar wounds, and for continued protection of the healed wound and the foot to include appropriate shoes, insoles, and monitoring of inflammation. |
strong |
High |
[93] |
- Research Needs
1. Assess the economic benefits of training vascular specialists in open and endovascular techniques and if this lowers the complications rate and saves money
2. Is open surgery a really cheaper option or the longer hospitalization and complications will make it most expensive than endovascular techniques
- Clinical indicators for monitoring
Any patient with CLTI should have the following :
1. ABI
2. Duplex ultrasound
3. CTA if revascularization is planned
- Updating the guideline
To keep these recommendations up to date and ensure its validity it will be periodically updated. This will be done whenever a strong new evidence is available and necessitates updation.
- References
[1] J. R. W. Brownrigg et al., “Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: a systematic review,” Diabetes Metab Res Rev, vol. 32 Suppl 1, pp. 128–135, Jan. 2016, doi: 10.1002/DMRR.2704.
[2] J. C. De Graaff, D. T. Ubbink, D. A. Legemate, J. G. P. Tijssen, and M. J. H. M. Jacobs, “Evaluation of toe pressure and transcutaneous oxygen measurements in management of chronic critical leg ischemia: A diagnostic randomized clinical trial,” J Vasc Surg, vol. 38, no. 3, pp. 528–534, Sep. 2003, doi: 10.1016/S0741-5214(03)00414-2.
[3] Z. Wang et al., “A systematic review and meta-analysis of tests to predict wound healing in diabetic foot,” J Vasc Surg, vol. 63, no. 2, pp. 29S-36S.e2, Feb. 2016, doi: 10.1016/j.jvs.2015.10.004.
[4] A. Hingorani et al., “A comparison of magnetic resonance angiography, contrast arteriography, and duplex arteriography for patients undergoing lower extremity revascularization,” Ann Vasc Surg, vol. 18, no. 3, pp. 294–301, 2004, doi: 10.1007/S10016-004-0039-0.
[5] E. Larch et al., “Value of color duplex sonography for evaluation of tibioperoneal arteries in patients with femoropopliteal obstruction: A prospective comparison with anterograde intraarterial digital subtraction angiography,” 1997.
[6] M. E. A. P. M. Adriaensen et al., “Peripheral arterial disease: therapeutic confidence of CT versus digital subtraction angiography and effects on additional imaging recommendations,” Radiology, vol. 233, no. 2, pp. 385–391, Nov. 2004, doi: 10.1148/RADIOL.2331031595.
[7] A. P. Hingorani et al., “Limitations of and lessons learned from clinical experience of 1,020 duplex arteriography,” Vascular, vol. 16, no. 3, pp. 147–153, May 2008, doi: 10.2310/6670.2008.00014.
[8] R. Collins et al., “A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic,” researchonline.lshtm.ac.ukR Collins, G Cranny, J Burch, R Aguiar-Ibanez, D Craig, K Wright, E Berry, M GoughHealth technology assessment (Winchester, England), 2007•researchonline.lshtm.ac.uk, vol. 11, no. 20, 2007, Accessed: Apr. 18, 2024. [Online]. Available: https://researchonline.lshtm.ac.uk/id/eprint/9259/
[9] “Long-term mortality and its predictors in patients with critical leg ischaemia. The I.C.A.I. Group (Gruppo di Studio dell’Ischemia Cronica Critica degli Arti Inferiori). The Study Group of Criticial Chronic Ischemia of the Lower Exremities - PubMed.” Accessed: Apr. 20, 2024. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/9314849/
[10] E. J. Armstrong et al., “Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease,” J Vasc Surg, vol. 60, no. 6, pp. 1565–1571, 2014, doi: 10.1016/J.JVS.2014.08.064.
[11] E. Faglia et al., “Effectiveness of combined therapy with angiotensin-converting enzyme inhibitors and statins in reducing mortality in diabetic patients with critical limb ischemia: an observational study,” Diabetes Res Clin Pract, vol. 103, no. 2, pp. 292–297, 2014, doi: 10.1016/J.DIABRES.2013.12.060.
[12] C. Baigent, C. Sudlow, R. Collins, and R. Peto, “Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients,” BMJ, vol. 324, no. 7329, pp. 71–86, Jan. 2002, doi: 10.1136/BMJ.324.7329.71.
[13] M. Gent, “A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee,” Lancet, vol. 348, no. 9038, pp. 1329–1339, Nov. 1996, doi: 10.1016/S0140-6736(96)09457-3.
[14] W. R. Hiatt et al., “Ticagrelor versus Clopidogrel in Symptomatic Peripheral Artery Disease,” N Engl J Med, vol. 376, no. 1, pp. 32–40, Jan. 2017, doi: 10.1056/NEJMOA1611688.
[15] S. S. ; Bosch et al., “Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial,” thelancet.comSS Anand, J Bosch, JW Eikelboom, SJ Connolly, R Diaz, P Widimsky, V Aboyans, M AlingsThe Lancet, 2018•thelancet.com, doi: 10.1016/S0140-6736(17)32409-1.
[16] P. P. Aung, H. G. Maxwell, R. G. Jepson, J. F. Price, and G. C. Leng, “Lipid-lowering for peripheral arterial disease of the lower limb,” Cochrane Database Syst Rev, vol. 2007, no. 4, 2007, doi: 10.1002/14651858.CD000123.PUB2.
[17] T. Meade, R. Zuhrie, C. Cook, and J. Cooper, “Bezafibrate in men with lower extremity arterial disease: randomised controlled trial,” BMJ, vol. 325, no. 7373, pp. 1139–1141, Nov. 2002, doi: 10.1136/BMJ.325.7373.1139.
[18] E. J. Mills et al., “Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40 000 patients,” Eur Heart J, vol. 32, no. 11, pp. 1409–1415, Jun. 2011, doi: 10.1093/EURHEARTJ/EHR035.
[19] F. Rodriguez, D. J. Maron, J. W. Knowles, S. S. Virani, S. Lin, and P. A. Heidenreich, “Association Between Intensity of Statin Therapy and Mortality in Patients With Atherosclerotic Cardiovascular Disease,” JAMA Cardiol, vol. 2, no. 1, pp. 47–54, Jan. 2017, doi: 10.1001/JAMACARDIO.2016.4052.
[20] S. C. Palmer et al., “Comparison of Clinical Outcomes and Adverse Events Associated With Glucose-Lowering Drugs in Patients With Type 2 Diabetes: A Meta-analysis,” JAMA, vol. 316, no. 3, pp. 313–324, Jul. 2016, doi: 10.1001/JAMA.2016.9400.
[21] S. Nawaz, T. Cleveland, P. A. Gaines, and P. Chan, “Clinical risk associated with contrast angiography in metformin treated patients: a clinical review,” Clin Radiol, vol. 53, no. 5, pp. 342–344, 1998, doi: 10.1016/S0009-9260(98)80005-6.
[22] S. K. Goergen, G. Rumbold, G. Compton, and C. Harris, “Systematic review of current guidelines, and their evidence base, on risk of lactic acidosis after administration of contrast medium for patients receiving metformin,” Radiology, vol. 254, no. 1, pp. 261–269, Jan. 2010, doi: 10.1148/RADIOL.09090690.
[23] F. Stacul et al., “Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines,” Eur Radiol, vol. 21, no. 12, pp. 2527–2541, 2011, doi: 10.1007/S00330-011-2225-0.
[24] D. L. Cull et al., “An early validation of the Society for Vascular Surgery lower extremity threatened limb classification system,” J Vasc Surg, vol. 60, no. 6, pp. 1535–1542, 2014, doi: 10.1016/J.JVS.2014.08.107.
[25] L. X. Zhan, B. C. Branco, D. G. Armstrong, and J. L. Mills, “The Society for Vascular Surgery lower extremity threatened limb classification system based on Wound, Ischemia, and foot Infection (WIfI) correlates with risk of major amputation and time to wound healing,” J Vasc Surg, vol. 61, no. 4, pp. 939–944, Apr. 2015, doi: 10.1016/J.JVS.2014.11.045.
[26] M. W. Causey et al., “Society for Vascular Surgery limb stage and patient risk correlate with outcomes in an amputation prevention program,” J Vasc Surg, vol. 63, no. 6, pp. 1563e2-1573.e2, Jun. 2016, doi: 10.1016/J.JVS.2016.01.011.
[27] J. D. Darling et al., “Predictive ability of the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system following infrapopliteal endovascular interventions for critical limb ischemia,” J Vasc Surg, vol. 64, no. 3, pp. 616–622, Sep. 2016, doi: 10.1016/J.JVS.2016.03.417.
[28] W. P. Robinson et al., “Society for Vascular Surgery Wound, Ischemia, foot Infection (WIfI) score correlates with the intensity of multimodal limb treatment and patient-centered outcomes in patients with threatened limbs managed in a limb preservation center,” J Vasc Surg, vol. 66, no. 2, pp. 488-498.e2, Aug. 2017, doi: 10.1016/J.JVS.2017.01.063.
[29] A. M. Abu Dabrh et al., “The natural history of untreated severe or critical limb ischemia,” J Vasc Surg, vol. 62, no. 6, pp. 1642-1651.e3, Dec. 2015, doi: 10.1016/J.JVS.2015.07.065.
[30] T. R. S, M. D. Ingegno, L. Carlton, T. C. Flynn, and J. M. Seeger, “Limb-threatening ischemia due to multilevel arterial occlusive disease. Simultaneous or staged inflow/outflow revascularization,” Ann Surg, vol. 221, no. 5, pp. 498–506, May 1995, doi: 10.1097/00000658-199505000-00007.
[31] K. R. Wengerter, F. J. Veith, S. K. Gupta, E. Ascer, and S. P. Rivers, “Influence of vein size (diameter) on infrapopliteal reversed vein graft patency,” J Vasc Surg, vol. 11, no. 4, pp. 525–531, Apr. 1990, doi: 10.1067/MVA.1990.18327.
[32] A. Schanzer et al., “Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial,” J Vasc Surg, vol. 46, no. 6, pp. 1180–1190, Dec. 2007, doi: 10.1016/J.JVS.2007.08.033.
[33] Z. G, U. H, and T. V, “Sequential aortofemoropopliteal/distal bypass for treatment of critical lower-limb ischaemia,” Cardiovasc Surg, vol. 3, no. 6, pp. 671–678, Dec. 1995, doi: 10.1016/0967-2109(96)82868-7.
[34] T. R. S, M. D. Ingegno, L. Carlton, T. C. Flynn, and J. M. Seeger, “Limb-threatening ischemia due to multilevel arterial occlusive disease. Simultaneous or staged inflow/outflow revascularization,” Ann Surg, vol. 221, no. 5, pp. 498–506, May 1995, doi: 10.1097/00000658-199505000-00007.
[35] Z. G, U. H, and T. V, “Sequential aortofemoropopliteal/distal bypass for treatment of critical lower-limb ischaemia,” Cardiovasc Surg, vol. 3, no. 6, pp. 671–678, Dec. 1995, doi: 10.1016/0967-2109(96)82868-7.
[36] V. Jongkind, G. J. M. Akkersdijk, K. K. Yeung, and W. Wisselink, “A systematic review of endovascular treatment of extensive aortoiliac occlusive disease,” J Vasc Surg, vol. 52, no. 5, pp. 1376–1383, 2010, doi: 10.1016/J.JVS.2010.04.080.
[37] W. Ye, C. W. Liu, J. B. Ricco, K. Mani, R. Zeng, and J. Jiang, “Early and late outcomes of percutaneous treatment of TransAtlantic Inter-Society Consensus class C and D aorto-iliac lesions,” J Vasc Surg, vol. 53, no. 6, pp. 1728–1737, 2011, doi: 10.1016/J.JVS.2011.02.005.
[38] K. Deloose et al., “Primary stenting is nowadays the gold standard treatment for TASC II A & B iliac lesions: the definitive MISAGO 1-year results,” J Cardiovasc Surg (Torino), vol. 58, no. 3, pp. 416–421, Jun. 2017, doi: 10.23736/S0021-9509.17.08303-3.
[39] J. B. Ricco and H. Probst, “Long-term results of a multicenter randomized study on direct versus crossover bypass for unilateral iliac artery occlusive disease,” J Vasc Surg, vol. 47, no. 1, 2008, doi: 10.1016/J.JVS.2007.08.050.
[40] K. W. H. Chiu, R. S. M. Davies, P. G. Nightingale, A. W. Bradbury, and D. J. Adam, “Review of direct anatomical open surgical management of atherosclerotic aorto-iliac occlusive disease,” Eur J Vasc Endovasc Surg, vol. 39, no. 4, pp. 460–471, Apr. 2010, doi: 10.1016/J.EJVS.2009.12.014.
[41] J. E. Indes et al., “Clinical outcomes of 5358 patients undergoing direct open bypass or endovascular treatment for aortoiliac occlusive disease: a systematic review and meta-analysis,” J Endovasc Ther, vol. 20, no. 4, pp. 443–455, Aug. 2013, doi: 10.1583/13-4242.1.
[42] J. L. Kang, V. I. Patel, M. F. Conrad, G. M. LaMuraglia, T. K. Chung, and R. P. Cambria, “Common femoral artery occlusive disease: contemporary results following surgical endarterectomy,” J Vasc Surg, vol. 48, no. 4, 2008, doi: 10.1016/J.JVS.2008.05.025.
[43] E. Ballotta, M. Gruppo, F. Mazzalai, and G. Da Giau, “Common femoral artery endarterectomy for occlusive disease: an 8-year single-center prospective study,” Surgery, vol. 147, no. 2, pp. 268–274, Feb. 2010, doi: 10.1016/J.SURG.2009.08.004.
[44] R. W. Chang, P. P. Goodney, J. H. Baek, B. W. Nolan, E. M. Rzucidlo, and R. J. Powell, “Long-term results of combined common femoral endarterectomy and iliac stenting/stent grafting for occlusive disease,” J Vasc Surg, vol. 48, no. 2, pp. 362–367, Aug. 2008, doi: 10.1016/J.JVS.2008.03.042.
[45] F. Baumann et al., “Endovascular treatment of common femoral artery obstructions,” J Vasc Surg, vol. 53, no. 4, pp. 1000–1006, Apr. 2011, doi: 10.1016/J.JVS.2010.10.076.
[46] R. F. Bonvini et al., “Endovascular treatment of common femoral artery disease: medium-term outcomes of 360 consecutive procedures,” J Am Coll Cardiol, vol. 58, no. 8, pp. 792–798, Aug. 2011, doi: 10.1016/J.JACC.2011.01.070.
[47] Y. Gouëffic et al., “Stenting or Surgery for De Novo Common Femoral Artery Stenosis,” JACC Cardiovasc Interv, vol. 10, no. 13, pp. 1344–1354, Jul. 2017, doi: 10.1016/J.JCIN.2017.03.046.
[48] J. J. Siracuse et al., “Endovascular treatment of the common femoral artery in the Vascular Quality Initiative,” J Vasc Surg, vol. 65, no. 4, pp. 1039–1046, Apr. 2017, doi: 10.1016/J.JVS.2016.10.078.
[49] J. Almasri et al., “A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia,” J Vasc Surg, vol. 68, no. 2, pp. 624–633, Aug. 2018, doi: 10.1016/J.JVS.2018.01.066.
[50] N. Azuma, H. Uchida, T. Kokubo, A. Koya, N. Akasaka, and T. Sasajima, “Factors influencing wound healing of critical ischaemic foot after bypass surgery: is the angiosome important in selecting bypass target artery?,” Eur J Vasc Endovasc Surg, vol. 43, no. 3, pp. 322–328, Mar. 2012, doi: 10.1016/J.EJVS.2011.12.001.
[51] B. E. Sumpio, R. O. Forsythe, K. R. Ziegler, J. G. Van Baal, M. J. A. Lepantalo, and R. J. Hinchliffe, “Clinical implications of the angiosome model in peripheral vascular disease,” J Vasc Surg, vol. 58, no. 3, pp. 814–826, Sep. 2013, doi: 10.1016/J.JVS.2013.06.056.
[52] F. Biancari and T. Juvonen, “Angiosome-targeted lower limb revascularization for ischemic foot wounds: systematic review and meta-analysis,” Eur J Vasc Endovasc Surg, vol. 47, no. 5, pp. 517–522, 2014, doi: 10.1016/J.EJVS.2013.12.010.
[53] K. J. Chae and J. Y. Shin, “Is Angiosome-Targeted Angioplasty Effective for Limb Salvage and Wound Healing in Diabetic Foot? : A Meta-Analysis,” PLoS One, vol. 11, no. 7, Jul. 2016, doi: 10.1371/JOURNAL.PONE.0159523.
[54] H. Jongsma, J. A. Bekken, G. P. Akkersdijk, S. E. Hoeks, H. J. Verhagen, and B. Fioole, “Angiosome-directed revascularization in patients with critical limb ischemia,” J Vasc Surg, vol. 65, no. 4, pp. 1208-1219.e1, Apr. 2017, doi: 10.1016/J.JVS.2016.10.100.
[55] M. Schillinger et al., “Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery,” N Engl J Med, vol. 354, no. 18, pp. 1879–1888, May 2006, doi: 10.1056/NEJMOA051303.
[56] R. R. Saxon, M. D. Dake, R. L. Volgelzang, B. T. Katzen, and G. J. Becker, “Randomized, multicenter study comparing expanded polytetrafluoroethylene-covered endoprosthesis placement with percutaneous transluminal angioplasty in the treatment of superficial femoral artery occlusive disease,” J Vasc Interv Radiol, vol. 19, no. 6, pp. 823–832, Jun. 2008, doi: 10.1016/J.JVIR.2008.02.008.
[57] M. D. Dake et al., “Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results,” Circ Cardiovasc Interv, vol. 4, no. 5, pp. 495–504, Oct. 2011, doi: 10.1161/CIRCINTERVENTIONS.111.962324.
[58] K. Rosenfield et al., “Trial of a Paclitaxel-Coated Balloon for Femoropopliteal Artery Disease,” N Engl J Med, vol. 373, no. 2, pp. 145–153, Jul. 2015, doi: 10.1056/NEJMOA1406235.
[59] J. L. Mills, R. M. Fujitani, and S. M. Taylor, “Contribution of routine intraoperative completion arteriography to early infrainguinal bypass patency,” Am J Surg, vol. 164, no. 5, pp. 506–511, 1992, doi: 10.1016/S0002-9610(05)81190-0.
[60] D. F. Bandyk, J. L. Mills, V. Gahtan, and G. E. Esses, “Intraoperative duplex scanning of arterial reconstructions: fate of repaired and unrepaired defects,” J Vasc Surg, vol. 20, no. 3, pp. 426–433, 1994, doi: 10.1016/0741-5214(94)90142-2.
[61] V. K. L. Karanth, T. K. Karanth, and L. Karanth, “Lumbar sympathectomy techniques for critical lower limb ischaemia due to non-reconstructable peripheral arterial disease,” Cochrane Database Syst Rev, vol. 12, no. 12, Dec. 2016, doi: 10.1002/14651858.CD011519.PUB2.
[62] V. Vietto, J. V. A. Franco, V. Saenz, D. Cytryn, J. Chas, and A. Ciapponi, “Prostanoids for critical limb ischaemia,” Cochrane Database Syst Rev, vol. 1, no. 1, 2018, doi: 10.1002/14651858.CD006544.PUB3.
[63] F. B. Smith, A. Bradbury, and G. Fowkes, “Intravenous naftidrofuryl for critical limb ischaemia,” Cochrane Database of Systematic Reviews, Jul. 2012, doi: 10.1002/14651858.CD002070.PUB2/ABSTRACT.
[64] P. Kranke, M. H. Bennett, M. Martyn-St James, A. Schnabel, S. E. Debus, and S. Weibel, “Hyperbaric oxygen therapy for chronic wounds,” Cochrane Database Syst Rev, vol. 2015, no. 6, Jun. 2015, doi: 10.1002/14651858.CD004123.PUB4.
[65] F. L. Game et al., “Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: a systematic review,” Diabetes Metab Res Rev, vol. 32 Suppl 1, pp. 154–168, Jan. 2016, doi: 10.1002/DMRR.2707.
[66] K. T. B. Santema et al., “Hyperbaric Oxygen Therapy in the Treatment of Ischemic Lower- Extremity Ulcers in Patients With Diabetes: Results of the DAMO2CLES Multicenter Randomized Clinical Trial,” Diabetes Care, vol. 41, no. 1, pp. 112–119, Jan. 2018, doi: 10.2337/DC17-0654.
[67] S. M. O. Peeters Weem, M. Teraa, G. J. De Borst, M. C. Verhaar, and F. L. Moll, “Bone Marrow derived Cell Therapy in Critical Limb Ischemia: A Meta-analysis of Randomized Placebo Controlled Trials,” Eur J Vasc Endovasc Surg, vol. 50, no. 6, pp. 775–783, Dec. 2015, doi: 10.1016/J.EJVS.2015.08.018.
[68] M. Elsherif, W. Tawfick, P. Canning, N. Hynes, and S. Sultan, “Quality of time spent without symptoms of disease or toxicity of treatment for transmetatarsal amputation versus digital amputation in diabetic patients with digital gangrene,” Vascular, vol. 26, no. 2, pp. 142–150, Apr. 2018, doi: 10.1177/1708538117718108.
[69] H. Aziz et al., “The influence of do-not-resuscitate status on the outcomes of patients undergoing emergency vascular operations,” J Vasc Surg, vol. 61, no. 6, pp. 1538–1542, Jun. 2015, doi: 10.1016/J.JVS.2014.11.087.
[70] J. J. Siracuse et al., “Impact of ‘Do Not Resuscitate’ Status on the Outcome of Major Vascular Surgical Procedures,” Ann Vasc Surg, vol. 29, no. 7, pp. 1339–1345, Oct. 2015, doi: 10.1016/J.AVSG.2015.05.014.
[71] A. B. Reed, C. Delvecchio, and J. S. Giglia, “Major lower extremity amputation after multiple revascularizations: was it worth it?,” Ann Vasc Surg, vol. 22, no. 3, pp. 335–340, May 2008, doi: 10.1016/J.AVSG.2007.07.039.
[72] D. L. Rollins, J. B. Towne, V. M. Bernhard, and P. L. Baum, “Isolated profundaplasty for limb salvage,” J Vasc Surg, vol. 2, no. 4, pp. 585–590, Jul. 1985, doi: 10.1067/MVA.1985.AVS0020585.
[73] B. J. Moran, P. Buttenshaw, M. Mulcahy, and K. P. Robinson, “Through‐knee amputation in high‐risk patients with vascular disease: Indications, complications and rehabilitation,” British Journal of Surgery, vol. 77, no. 10, pp. 1118–1120, 1990, doi: 10.1002/bjs.1800771014.
[74] S. M. Taylor et al., “‘Successful outcome’ after below-knee amputation: an objective definition and influence of clinical variables,” Am Surg, vol. 74, no. 7, pp. 607–612, Jul. 2008, doi: 10.1177/000313480807400707.
[75] L. Bradley and S. G. B. Kirker, “Secondary prevention of arteriosclerosis in lower limb vascular amputees: a missed opportunity,” Eur J Vasc Endovasc Surg, vol. 32, no. 5, pp. 491–493, Nov. 2006, doi: 10.1016/J.EJVS.2006.07.005.
[76] J. D. Glaser et al., “Fate of the contralateral limb after lower extremity amputation,” J Vasc Surg, vol. 58, no. 6, 2013, doi: 10.1016/J.JVS.2013.06.055.
[77] T. A. Abbruzzese et al., “Statin therapy is associated with improved patency of autogenous infrainguinal bypass grafts,” J Vasc Surg, vol. 39, no. 6, pp. 1178–1185, Jun. 2004, doi: 10.1016/j.jvs.2003.12.027.
[78] P. K. Henke et al., “Patients undergoing infrainguinal bypass to treat atherosclerotic vascular disease are underprescribed cardioprotective medications: Effect on graft patency, limb salvage, and mortality,” J Vasc Surg, vol. 39, no. 2, pp. 357–365, 2004, doi: 10.1016/j.jvs.2003.08.030.
[79] J. Brown, A. Lethaby, H. Maxwell, A. J. Wawrzyniak, and M. H. Prins, “Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery,” Cochrane Database Syst Rev, no. 4, 2008, doi: 10.1002/14651858.CD000535.PUB2.
[80] R. Bedenis, A. Lethaby, H. Maxwell, S. Acosta, and M. H. Prins, “Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery,” Cochrane Database Syst Rev, vol. 2015, no. 2, Feb. 2015, doi: 10.1002/14651858.CD000535.PUB3.
[81] B. D. Suckow et al., “Statin therapy after infrainguinal bypass surgery for critical limb ischemia is associated with improved 5-year survival,” J Vasc Surg, vol. 61, no. 1, pp. 126-133.e1, Jan. 2015, doi: 10.1016/J.JVS.2014.05.093.
[82] S. D. Hobbs and A. W. Bradbury, “Smoking cessation strategies in patients with peripheral arterial disease: An evidence-based approach,” European Journal of Vascular and Endovascular Surgery, vol. 26, no. 4, pp. 341–347, Oct. 2003, doi: 10.1016/S1078-5884(03)00356-3.
[83] E. M. Willigendael, J. A. W. Teijink, M. L. Bartelink, R. J. G. Peters, H. R. Büller, and M. H. Prins, “Smoking and the patency of lower extremity bypass grafts: A meta-analysis,” J Vasc Surg, vol. 42, no. 1, pp. 67–74, 2005, doi: 10.1016/j.jvs.2005.03.024.
[84] B. JJ et al., “Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial,” J Vasc Surg, vol. 52, no. 4, pp. 825-833.e2, 2010, doi: 10.1016/J.JVS.2010.04.027.
[85] A. A. Gassman et al., “Aspirin usage is associated with improved prosthetic infrainguinal bypass graft patency,” Vascular, vol. 22, no. 2, pp. 105–111, 2014, doi: 10.1177/1708538112473977.
[86] D. L. Bhatt et al., “Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events,” N Engl J Med, vol. 354, no. 16, pp. 1706–1717, Apr. 2006, doi: 10.1056/NEJMOA060989.
[87] G. Tepe et al., “Management of peripheral arterial interventions with mono or dual antiplatelet therapy--the MIRROR study: a randomised and double-blinded clinical trial,” Eur Radiol, vol. 22, no. 9, pp. 1998–2006, Sep. 2012, doi: 10.1007/S00330-012-2441-2.
[88] F. F. Strobl et al., “Twelve-month results of a randomized trial comparing mono with dual antiplatelet therapy in endovascularly treated patients with peripheral artery disease,” J Endovasc Ther, vol. 20, no. 5, pp. 699–706, Oct. 2013, doi: 10.1583/13-4275MR.1.
[89] K. Cassar, I. Ford, M. Greaves, P. Bachoo, and J. Brittenden, “Randomized clinical trial of the antiplatelet effects of aspirin-clopidogrel combination versus aspirin alone after lower limb angioplasty,” Br J Surg, vol. 92, no. 2, pp. 159–165, Feb. 2005, doi: 10.1002/BJS.4810.
[90] J. L. Mills, C. L. Wixon, D. C. James, J. Devine, A. Westerband, and J. D. Hughes, “The natural history of intermediate and critical vein graft stenosis: recommendations for continued surveillance or repair,” J Vasc Surg, vol. 33, no. 2, pp. 273–280, 2001, doi: 10.1067/MVA.2001.112701.
[91] G. J. Landry, G. L. Moneta, L. M. Taylor, J. M. Edwards, R. A. Yeager, and J. M. Porter, “Long-term outcome of revised lower-extremity bypass grafts,” J Vasc Surg, vol. 35, no. 1, pp. 56–63, 2002, doi: 10.1067/mva.2002.120040.
[92] L. L. Nguyen et al., “Infrainguinal vein bypass graft revision: Factors affecting long-term outcome,” J Vasc Surg, vol. 40, no. 5, pp. 916–923, 2004, doi: 10.1016/j.jvs.2004.08.038.
[93] T. Elraiyah et al., “A systematic review and meta-analysis of off-loading methods for diabetic foot ulcers,” J Vasc Surg, vol. 63, no. 2 Suppl, pp. 59S-68S.e2, Feb. 2016, doi: 10.1016/J.JVS.2015.10.006.
[94] A. J. Boulton, L. Vileikyte, G. Ragnarson-Tennvall, and J. Apelqvist, “The global burden of diabetic foot disease,” Lancet, vol. 366, no. 9498, pp. 1719–1724, Nov. 2005, doi: 10.1016/S0140-6736(05)67698-2.
- Annexes
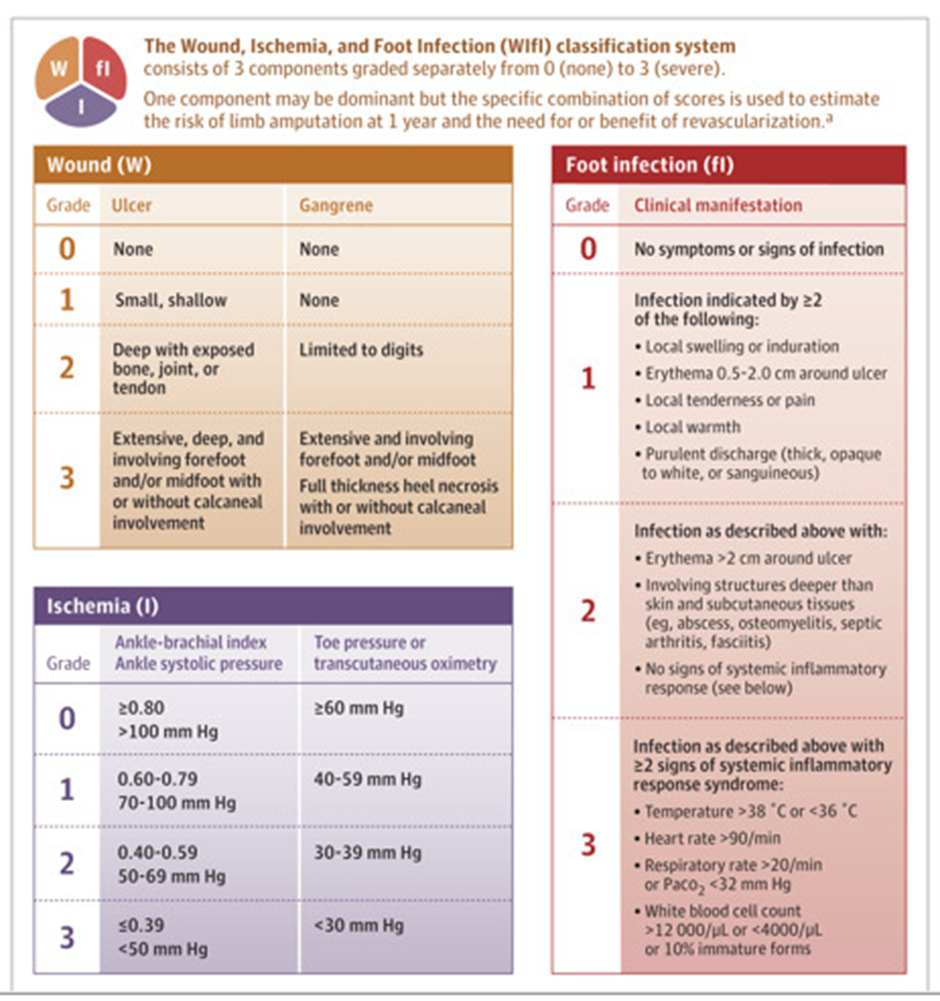
Figure from JAMA 2023 Jul 3;330 1 [94]