Microsporidiosis
| Site: | EHC | Egyptian Health Council |
| Course: | Aquatic animal medicine Guidelines |
| Book: | Microsporidiosis |
| Printed by: | Guest user |
| Date: | Tuesday, 24 December 2024, 2:55 AM |
Description
"last update:24 June 2024"
- Committee
We would like to acknowledge the committee of National Egyptian Guidelines for Veterinary Medical Interventions, Egyptian Health Council for adapting this guideline.
Executive Chief of the Egyptian Health Council: Prof. Dr Mohamed Mustafa Lotief.
Committee Chair: Prof. Ahmed M Byomi
The Rapporteur of the Committee: Prof. Dr Mohamed Mohamedy Ghanem.
Committee Members: Prof. Nabil yassien; Prof. Ashraf Aldesoky Shamaa; Prof. Amany Abbass; Prof. Dalia Mansour; Dr Essam Sobhy.
Authors: Mohamed Faisal1,2; Amany A. Abbass1 ; Adel A. Shaheen1; Amel M. El Asely1; Eman A. Abd El-Gawad1; Hiam S. Elabd1; Aya F. Matter1; Hadeer A. Youssef1, and Amira M. El-Daim1.
1Department of Aquatic Animal Medicine, Faculty of Veterinary Medicine, Benha University, Egypt.
2College of Veterinary Medicine, Michigan State University, USA.
- Scope
A disease caused by an obligate eukaryotic intracellular pathogen recently classified as a member of kingdom fungi infecting wild and farmed fish. The disease reduces both the productivity and marketability of infected fish. The pathogen infects a wide range of animals and has a zoonotic importance.
- Summary
Microsporidiosis is a disease caused by Microsporidia spp. which is described as tiny eukaryotic organisms belonging to the kingdom fungi. The pathogen can invade various animals, such as humans, mammals, birds, fish, and insects. Microsporidia have some distinct physical characteristics with their spores resistant to damage, varying in size, and possessing a unique organelle called the polar tubule or polar filament. The pathogen is transmitted horizontally as well as vertically. Diagnosis primarily relies on spore structure, clinical signs, staining of xenoma contents, histological examination, transmission electron microscopy, and molecular analysis.
- Introduction
Microsporidian infections represent a constant threat to aquaculture. These parasites are widely distributed in seawater, freshwater and estuaries causing major losses. They are a widespread and diverse group that infects many species, from protists to humans. Horizontal transmission can happen between hosts that are related or unrelated to the same or different life stages and from the same or different species. On the other hand, vertical transmission occurs within a host family, where parasites are passed down through generations of hosts. (Dunn and Smith, 2001). Microsporidia commonly transmit through the ingestion of spores, which infect the gut and spread to other tissues. Transmission and virulence are strongly linked, with high parasite burden and pathogenicity in horizontal transmission, and low virulence in vertical transmission.
In Egypt, various microsporidia species have been identified from marine and freshwater fishes e.g., Red porgy, Pagrus pagrus, Gilthead seabream, Sparus aurata, Flathead grey mullet Mugil cephalus, and African sharptooth catfish Clarias gariepinus (Eissa 1995 &2002; Shehab El-Din, 2008; Morsy et al., 2013; Abd Rabo, 2017, Abd El-Lateif and Torra 2020)
- Etiological agents
◾Microsporidia are currently related to Fungi, previously thought to be Protozoa (Capella-Gutiérrez et al., 2012).
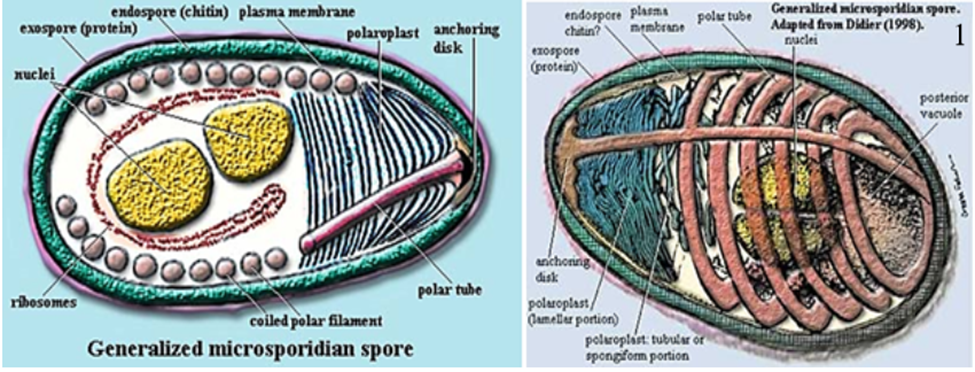
◾Spores are the infective stage which invades host cells through a specialized invasion apparatus; the polar tube (Fig. 1) (Han et al., 2020).
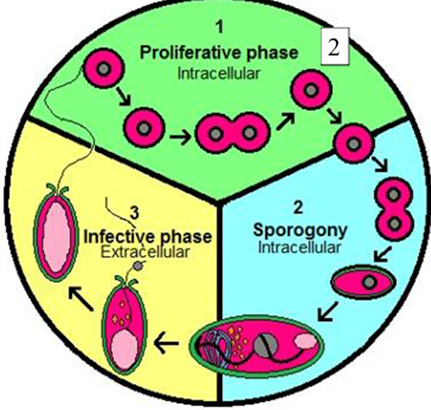
◾The life cycle of microsporidia comprises three phases: Phase I is the extracellular phase containing mature spores. Phase II is the first phase of intracellular development where microsporidian organisms increase in number. Phase III is the sporogonic phase, which leads to spore formation (Fig. 2).
◾The spore resists severe environmental conditions due to its thick chitinous layer (Yang et al., 2018). Spores are ovoid or ellipsoidal, ranging from 1 to 20 μm (Cali et al., 2017).
◾Phylum Microsporidia Balbiani 1882 includes more than two hundred genera and about 1300 species (Cali et al., 2017). Microsporidia can be characterized based solely on spore structure, including spore size, shape, and the number and position of polar capsules.
◾The fish-infecting genus is classified into five groups microsporidia. Group 1 is represented by the family Pleistophoridae. Group 2 is represented by the family Glugeidae. Group 3 comprises three clades: Loma and a hyperparasitic microsporidian from a myxosporean; Ichthyosporidium and Pseudoloma clade and the Loma acerinae clade. Group 4 contains two families, Spragueidae with the genus and Tetramicridae. Group 5 is represented by the family Enterocytozoonidae with the genus Nucleospora and the mammal-infecting genus Enterocytozoon (Lom and Nilsen, 2003).
◾The pathogen is mainly transmitted horizontally as well as vertically.
- Diagnosis
Clinical Signs
◾In C. gariepinus infected with Glugea sp. lesions characterized by whitish nodules (xenomas) embedded in kidney tissues containing viscous milky fluid (Abd Rabo, 2017). Some fish may carry parasites without demonstrating clinical signs, resulting in a subclinical infection. Nodules may be embedded in the connective tissue of the esophagus, intestinal submucosa, stomach, intestine and kidney (Fig. 3A, Abd Rabo 2017). In Saurida tumbil, Pleistophora aegyptiaca sp. nov. infection is associated with nodules (tumor like) in the peritoneal cavity, internal organs and within the skeletal muscle cells of fish produce grossly visible lesions (Fig. 3, Shehab El- Din, 2008). These nodules render the fish unmarketable (bdel-Ghaffar et al., 2012). Xenomas (cysts) may be embedded in all body organs, including muscles, liver, intestine, and stomach. Some marine fishes show white yellowish nodules caused by Glugea sp. and Pleistophora (Abdel-Mawla and Mohamed, 2010) whereas no nodules exist in Mugil cephalus.

Fig. 3: 3A) Xenomas embedded in the kidney as oval and small nodules. 3B) Saurida tumbil, showing Pleistophora aegyptiaca sp. nov. infection associated with nodules in the peritoneal cavity, internal organs and within the skeletal muscles.
▪️ Laboratory Diagnosis
Samples are taken by excising xenoma from affected organs which differ according to the pathogen species and the kind of infected fish.
▪️ Presumptive diagnosis
◾In fresh preparation from Yellow perch (Perca flavescens), spores appear oval to pyriform with an eccentrically located posterior vacuole and a sporophorous vesicle with several spores (arrowhead) of Heterosporis sutherlandae n. sp., (Fig. 4A) (Phelps et al., 2015). Wet mount from Glugea xenoma of C. gariepinus exhibits a typical egg-shaped spore of Glugea (Abd Rabo, 2017).
◾Staining xenoma contents with Geimsa stain revealed uninucleated meront (M) and dividing meront (D) (x40) (Fig. 4B) (Abd Rabo, 2017).
◾The microsporidian species can undergo spore discharge through the extrusion of the polar filament. This discharge can occur spontaneously (germination) or through incubation at various factors depending on the species. For instance, incubation at alkaline pH, acidic pH, or a pH shift from acid to alkaline or from alkaline to neutral. Dehydration through drying or hyperosmotic solutions followed by rehydration is another factor that can trigger spore discharge. Various cations including potassium, lithium, sodium, and cesium, and anions such as bromide, chloride, and iodide can also initiate spore discharge. Other factors that can trigger spore discharge include hydrogen peroxide, low dose ultraviolet radiation, and calcium ionophore A 23187 (Frixione et al., 1994. He et al., 1996).
▪️ Histological sections
◾Staining with conventional stains or Giemsa revealed that some spores appeared blue while others remained unstained.
◾Gram–Twort stain, both showing spores within the cytoplasm of degenerate epithelial cells (Fig. 4C) (Herreroet et al., 2020).
◾Staining with Calcofluor white fluorescent stain showing small (yellow arrows) and larger (white arrows) spores in the lamellar (Fig. 4D) (Herreroet al., 2020).
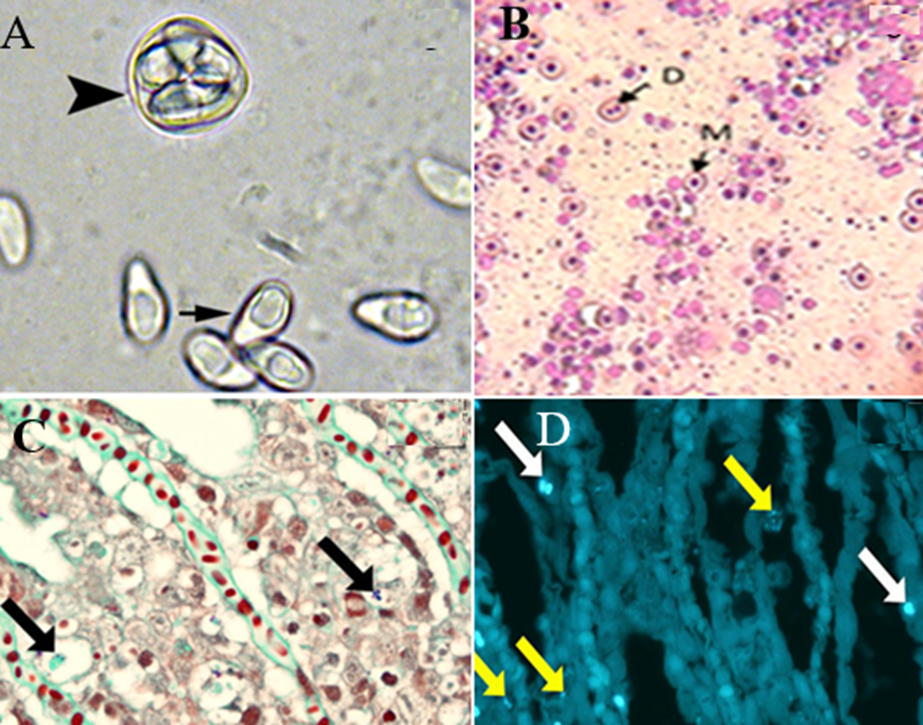
Fig. 4: 4A) spores appear oval to pyriform with an eccentrically located posterior vacuole (arrow) and a sporophorous vesicle with several spores (arrowhead) of Heterosporis sutherlandae n. sp. 4B) Staining xenoma contents with Geimsa revealed uninucleated meront (M) and dividing meront (D). 4C) Gram–Twort stain showing spores within the cytoplasm of degenerate epithelial cells. 4D) Calcofluor white fluorescent stain showing small (yellow arrows) and larger (white arrows) spores in the lamellar.
◾Transmission electron microscopy is a crucial research tool that is essential for a complete spore description.
▪️ Confirmatory diagnosis
◾PCR based assay; Samples are taken from the liver. The samples are collected from fish showing typical clinical signs. Liver samples are preserved in RNA later. DNA was extracted using the QIAamp DNA Mini Kit (Qiagen) following the manufacturer’s instructions. Fig. 5; The blasted sequence on the GenBank blast (NCBI) indicates that this sequence is related to fungus spp. (Alternaria Alternate) with similarity ~ 97%.

◾Molecular analysis of the rRNA genes including the ITS region of Glugea sp. uses species-specific designed primers.
◾Immunological methods Enzyme-linked immunosorbent assay (ELISA) and immunofluorescence assays can detect specific microsporidia antibodies in fish serum, indicating an infection.
◾Some species of microsporidia have been propagated in cell cultures.
- Control
Prevention
◾Microsporidia should be managed and prevented with improved hygiene.
◾It is crucial to separate tanks in the main facility to control the spread of microsporidia. These parasites can be transmitted through water and infected fish and can easily spread if there are mixing of fish in tanks.
Treatment
◾Although treatment is difficult fumagillin has proven to be effective for several microsporidia infections in some fish species.
Zoonotic importance
◾Out of the almost 1500 species known, only 17 can cause disease in humans. Four human-pathogenic microsporidia, namely Enterocytozoon bieneusi, Encephalitozoon intestinalis, Encephalitozoon hellem, and Encephalitozoon cuniculi, had been found in commercially harvested wild and farmed marine sources in Mediterranean coasts. Further research is required to better understand the potential role of fish in the spread of disease (Moratal et al., 2022).
◾N.B. Freezing and heating techniques are effective in destroying microsporidia spores. Fish processors can disinfect their hands and utensils by using 70% ethanol. (Leiro et al., 2012).
- References
Abdel-Ghaffar F, Bashtar A-R, Morsy K, Mehlhorn H, Al Quraishy S, AL-Rasheid K and Abdel-Gaber R. 2012. Morphological and molecular biological characterization of Pleistophora aegyptiaca sp. nov. infecting the Red Sea fish Saurida tumbil. Parasitol Res 110:741–752
Abdel Mawla HI and Mohamed SY. 2010. Studies on Microsporidiosis among some marine fishes and their associated pathological lesions. Assiut Vet. Med. J. Vol. 56 No. 125. 56- 67.
Ahmed NH, Caffara M, Sitjà-Bobadilla A, Fioravanti ML, Mazzone A, Aboulezz AS, Metwally, AM, Omar MA and Palenzuela OR. 2019. Detection of the intranuclear microsporidian Enterospora nucleophila in gilthead sea bream by in situ hybridization. J. Fish Dis.42, 809–815
Abd Rabo EA. 2017. Studies on some parasitic diseases affecting African catfish (Clarias gariepinus) from downstream El-Rahawy drain. M.V. Sc thesis (Fish Diseases and Management). Faculty of Veterinary Medicine, Benha University.
Capella-Gutiérrez S, Marcet-Houben M and Gabaldón T. 2012. Phylogenomics supports microsporidia as the earliest diverging clade of sequenced fungi. BMC Biol. 10:1. doi: 10.1186/1741-7007-10-47.
Dunn AM, Smith JE., 2001. Microsporidian life cycles and diversity: the relationship between virulence and transmission. Microbes and Infection, 3, 2001, 381−388 © 2001 Éditions scientifiques et médicales Elsevier SAS. All rights reserved S1286457901013946/RE
Eissa, IAM, 2002. Parasitic fish diseases in Egypt. Dar El-Nahda El-Arabia Publishing, 32 Abd El-Khalek St. Cairo, Egypt.
Eissa, IAM. 1995. Studies of parasitic dis-eases in marine Hake fish (Saurustumbil) for the first time in Egypt. Zag. Vet. J., 23, 4, 90-93.
Frixione E, Ruiz L, Undeen AH. 1994. Monovalent cations induce microsporidian spore germination in vitro. J Eukaryot Microbiol. 41: 464–468.
Han B, Takvorian PM and Weiss LM. 2020. Invasion of Host Cells by Microsporidia. Front. Microbiol. 11:172. doi: 10.3389/fmicb.2020.00172
He Q, Leitch GJ, Visvesvara GS, and Wallace S. 1996. Effects of nifedipine, metronidazole, and nitric oxide donors on spore germination and cell culture infection of the microsporidia Encephalitozoon hellem and Encephalitozoon intestinalis. Antimicrob. Agents Chemother. 40, 179–185. doi: 10.1128/aac.40.1.179.
Herrero AFP, Pflaum S, Matthews C, Jorge del-Pozo, Rodger HD, Dagleish MP and Thompson KD. 2020. Comparison of histologic methods for the detection of Desmozoon lepeophtherii spores in the gills of Atlantic salmon. Journal of Veterinary Diagnostic Investigation, Vol. 32(1) 142–146
Leiro, J. M., Piazzon, C., Domínguez, B., Mallo, N., & Lamas, J. (2012). Evaluation of some physical and chemical treatments for inactivating microsporidian spores isolated from fish. International Journal of Food Microbiology, 156(2), 152-160. https://doi.org/10.1016/j.ijfoodmicro.2012.03.017
Lom J, Nilsen F. 2003. Fish microsporidia: fine structural diversity and phylogeny. Int J Parasitol. 33(2):107-27. doi: 10.1016/s0020-7519(02)00252-7. PMID: 12633649.
Moratal, S., Magnet, A., Izquierdo, F., Del Águila, C., & Auxiliadora, M. (2022). Microsporidia in Commercially Harvested Marine Fish: A Potential Health Risk for Consumers. Animals, 13(16), 2673. https://doi.org/10.3390/ani13162673
Morsy K, Bashtar AR, Abdel-Ghaffar F, Al-Quraishy S. 2013. Morphological and phylogenetic description of a new xenoma-inducing microsporidian, Microsporidium aurata nov. sp., parasite of the gilthead seabream Sparus aurata from the Red Sea. Parasitol Res. 2013 Nov;112(11):3905-15. doi: 10.1007/s00436-013-3580-3.
Abd El-Lateif RSA and Torra DE. 2020. Parasitological and pathological studies on Microsporidian infection in African sharptooth catfish (Clarias gariepinus). Animal Health Research Journal, Vol. 8, No. 1, pp. 40-48.
Phelps NBD, Mor SK, Armién AG, Pelican KM, Goyal SM (2015) Description of the Microsporidian Parasite, Heterosporis sutherlandae n. sp., Infecting Fish in the Great Lakes Region, USA. PLoS ONE 10(8): e0132027. doi: 10.1371/journal.pone.0132027
Yang D, Pan L, Chen Z, Du H, Luo B, Luo J and Pan G.2018. The roles of microsporidia spore wall proteins in the spore wall formation and polar tube anchorage to spore wall during development and infection processes. Exp. Parasitol. 187, 93–100