Edwardsiellosis
| Site: | EHC | Egyptian Health Council |
| Course: | Aquatic animal medicine Guidelines |
| Book: | Edwardsiellosis |
| Printed by: | Guest user |
| Date: | Tuesday, 24 December 2024, 1:33 AM |
Description
"last update:9 June 2024"
- Committee
We would like to acknowledge the committee of National Egyptian Guidelines for Veterinary Medical Interventions, Egyptian Health Council for adapting this guideline.
Executive Chief of the Egyptian Health Council: Prof. Dr Mohamed Mustafa Lotief.
Committee Chair: Prof. Ahmed M Byomi
The Rapporteur of the Committee: Prof. Dr Mohamed Mohamedy Ghanem.
Committee Members: Prof.Nabil yassien; Prof. Ashraf Aldesoky Shamaa; Prof. Amany Abbass; Prof. Dalia Mansour; Dr Essam Sobhy.
Authors: Mohamed Faisal1,2; Amany A. Abbass1; Adel A. Shaheen1; Amel M. El Asely1; Eman A. Abd El-Gawad1; Hiam S. Elabd1; Aya F. Matter1; Hadeer A. Youssef1, and Amira M. El-Daim1.
1Department of Aquatic Animal Medicine, Faculty of Veterinary Medicine, Benha University, Egypt.
2College of Veterinary Medicine, Michigan State University, USA.
- Scope
Edwardsiella septicemia is a serious systemic bacterial infection caused by Edwardsiella piscicida/ tarda of the genus Edwardsiella and family Enterobacteriaceae. A wide range of hosts, including freshwater, brackish water, and marine aquatic species, are susceptible to the bacterium that causes Edwardsiellosis, which results in significant financial losses for aquaculture globally.
Synonyms of disease: emphesematous putrefactive disease of catfish, red disease of eels, and Edwardsiella septicemia of salmon, tilapia, and striped bass.
- Summary
Edwardsiellosis is an acute to chronic infection that greatly increases mortality, reduces fish marketability, and decreases total productivity with increase of production costs. It is reported that the ranges for mortality and morbidity are 5% to 30% and 5% to 70%, respectively. The disease is characterized by signs of septicemia including redness of the mouth and vent, exophthalmia, abdominal distension, necrotic abscesses in the muscle of putrid odor and grayish white nodules in internal organs. As well as it has been associated with intestinal and extraintestinal infection in humans. Many types of vaccine have been developed for disease prevention such as FKC, LPS, and ECP. In addition, oxytetracycline, florfenicol, and sulphadimethoxine-ormetoprim are the common antibiotics approved by FDA for treatment of Edwardsiellosis
- Introduction
Edwardsiella tarda (E. tarda) was first isolated from infected humans and animals (Ewing et al. 1965). The bacterium is a common ubiquitous pathogen, and wide variety of animals act as reservoirs of bacteria as well as it is a part of the normal gut microbiota of aquatic animals. E. tarda can be spread to vulnerable fish via skin or gill damage when contacted with contaminated water source (faeces, water or mud). Globally, E. piscicida/tarda is an important fish pathogen affecting wide range of fish species (Leung et al., 2019). In addition, it induces disease in other animals such as marine mammals, pigs, turtles, alligators, ostriches, skunks, snakes, and humans.
In Egypt, E. tarda was recorded in different freshwater fishes such as Nile tilapia, Oreochromius niloticus and common carp, Cyprinus carpio, African catfish, Clarias gariepinus (Noor El Deen et al., 2017; Algammal et al., 2022). Co-infection with Edwardsiella tarda and Edwardsiella anguillarum were also identified from Nile tilapia summer mortality syndrome (Elgendy et al., 2022).
- Etiological agent
▪️ Edwardsiella piscicida (old name Edwardsiella tarda (E. tarda) is a gram-negative rod-shaped bacterium (1 μm in diameter and 2–3 μm long) and motile.
▪️ The genus Edwardsiella has previously contained three species; E. tarda isolated from humans and animals, E. hoshinae isolated from reptiles and birds, and E. ictaluri isolated from channel and white catfish.
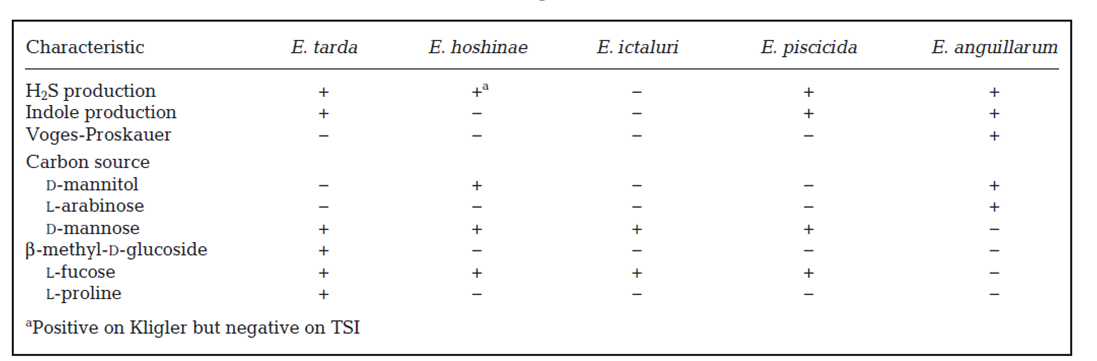
▪️ Recently, according to molecular and phylogenetic assay, the genus Edwardsiella was reclassified into five species; three fish pathogens (E. piscicida, E. anguillarum, and E. ictaluri) and two non-fish pathogens (E. tarda and E. hoshinae) (Buján et al., 2018).
▪️ E. piscicida is the new name for the previously isolated E. tarda which possesses the type III and type VI secretion systems (T3SS and T6SS). Meanwhile, E. tarda recovered from human or environment do not have any of the previous secretion system (Shao et al., 2018).
Table 1: Differential phenotypic characteristics among Edwardsiella species (Buján et al., 2018)
▪️ The severity of fish Edwardsiellosis has been influenced by many predisposing factors, such as environmental stressors (high temperature, high ammonia and carbon dioxide, low dissolved oxygen), high organic matter, heavy parasitic infestation, and improper use of antibiotic chemicals in aquaculture.
▪️ It was reported that the bacteria can be isolated from sterile pond water over 76 days at 20°C, indicating that the bacterium can remain alive for long time in the environment. E. tarda was isolated from both fresh and salt water, mud, and fouling material in nets. Moreover, it can stay alive at 0–4% sodium chloride, pH 4.0–10.0, and 14–45°C.
- Diagnosis
a-Clinical signs and lesions
▪️ The mortality rate of diseased fish may be acute or chronic depending on the degree of stressful environmental conditions that allow E. tarda infections to emerge.

▪️ Gross external lesions vary with species. In Nile tilapia the fish showed septicemic signs including haemorrhages on body surface and base of fins and congestion (Fig 1) (El-seedy et al., 2015), exophthalmia and cataracts (Fig 2 (Noor El Deen et al., 2017). Meanwhile, in striped bass, abscesses in internal organs were recorded, and necrotic abscesses in the muscle that emit a putrid odor when incised in channel catfish.
▪️ The disease in eels is known by two forms: the suppurative interstitial nephritis and the suppurative hepatitis forms (Miyazaki and Egusa 1976a and b).
▪️ Generally, petechiae and dermal ulceration, iridial haemorrhage, reddening of the mouth and vent, exophthalmia, ascites, splenomegaly, renomegaly, and intestinal haemorrhage have been recorded among diseased fishes (López‐Porras et al., 2021).
Note: The fish clinical signs are comparable to those of other bacterial diseases, such as motile aeromonas septicemia, vibriosis, Pseudomonas septicemia; streptococcosis and consequently, biochemical, and molecular approaches are endorsed for diagnosis of disease.
b-Laboratory diagnosis
▪️ Samples: liver, spleen, kidneys, and skin lesions of infected tissue from moribund or freshly dead fish are appropriate for bacteriological examination. Also, tissue specimens for histopathological analysis should be done.
I-Presumptive diagnostic assay
➡️ Isolation and identification
▪️ The bacterium is Gram-negative commonly motile but isolates from red seabream and yellowtail are non-motile (Matsuyama et al., 2005). The microorganism gives typical green colonies with black centers colonies on Edwardsiella isolation media (EIM) (Acharya et al., 2007) and producing indole from tryptone and an alkaline slant over an acid butt in triple sugar iron agar with production of H2S and gas.
▪️ The most important biochemical tests that classified E. tarda into two groups: wild type and biogroup 1, included mannitol and arabinose fermentation, ornithine decarboxylase, citrate utilization, and hydrogen sulfide production. The fermentation of arabinose, mannitol and sucrose is negative in wild-type E. tarda, but it is positive for H2S; meanwhile biogroup 1 are positive for the previous biochemical test. (Walton et al., 1993)
II-Histopathological examination
▪️ Naturally diseased Nile tilapia revealed hydropic degeneration, coagulative necrosis, multi-focal areas of hemorrhages between the hepatic cells (Noor El Deen et al., 2017).
▪️ Mononuclear meningoencephalitis, granulomatous hepatitis and interstitial nephritis, splenitis, hemorrhagic enteritis, hemorrhagic branchitis, and ulcerative dermatitis were also recorded (Griffin et al., 2019).
III-Confirmatory diagnosis
➡️ Serological techniques
▪️ Agglutination tests, enzyme linked immunosorbent assays (ELISA), and fluorescent antibody techniques are valuable diagnostic procedures for disease (Swain and Nayak, 2003). Park et al. (1983) based on O-antigen extracts classified E. tarda into serotypes A, B, C and D.
➡️ Molecular assay
▪️ There are many investigations documented that PCR-based techniques can provide accurate, sensitive, and differential diagnosis.
▪️ Fluorescence in situ hybridization (FISH) technique by Ootsubo et al. (2002), and high-performance capillary electrophoresis (HPCE) by Yu et al. (2004) has been used to detect bacteria in infected tissues and pond water.
▪️ Multiplex nested PCR has been carried out to identify the fish pathogens; A. hydrophila, Edwardsiella tarda, Photobacterium damselae and S. iniae (Chang et al., 2009).
▪️ Recombinase polymerase amplification combined with lateral flow strip (RPA-LF) was carried out to monitor E. piscicida infection (Jiang et al., 2022).
- Control of disease
➡️ Prevention
▪️ Good management practices is the best way to prevent E. tarda infections.
▪️ Several vaccine protocols have been developed to prevent edwardsiellosis, including formalin killed cells, Lipopolysaccharides, extracellular product, live attenuated E. tarda, avirulent E. tarda, ghost cells, outer membrane protein, recombinant proteins, recombinant protein-expressing cells, outer membrane vesicle, and DNA vaccines (Park et al., 2012).
▪️ Phytobiotics or plant-based products that have antimicrobial activity have been used for treatment of many bacterial diseases such as Ginger nanoparticles succeeded in preventing Edwardsiellosis in C. gariepinus (Korni et al., 2021).
▪️ Probiotics such as Enterococcus faecium, Bacillus subtilis, Lactobacillus acidophilus, and Saccharomyces cerevisiae oral administration improved fish survivability against edwardsiellosis (Essa et al. 2012).
➡️ Treatment
▪️ Sensitivity test should be carried out using the approved antibiotics US Food and Drug Administration (FDA) for incorporation in diets.
Ø Oxytetracycline can be used at 50 mg/kg body weight/ day for 12-14 days, followed by withdrawal period lasting for 21-day.
▪️ Sulphadimethoxine-ormetoprim in 5:1 ratio. 50-100 mg/kg body weight/ day for 5 days followed by withdrawal time for 3 days for skinned fish and 42 days for non-skinned fish before sold. Aquaflor® (florafenicol), ciprofloxacine and sulphadimethexine were also effective medicated antibacterial medicine in controlling edwardsiellosis (Noor El Deen et al., 2017).
➡️ Zoonotic importance
▪️ E. tarda is a zoonotic pathogen and humans become infected from either handling or eating infected fish. Salmonella-like gastroenteritis symptoms due to E. tarda infection have been reported (Schlenker and Surawicz, 2009).
▪️ Liver abscess, peritonitis, cellulitis, bacteremia, intra-abdominal abscess, tubo-ovarian abscess, mycotic aneurysm, and infection of biliary tract are examples of extra-intestinal edwardsiellosis (Wang et al., 2005). The most vulnerable persons to infection are those suffering from other diseases and having worsened immunity.
- References
Acharya M, Maiti N K, Mohanty S, Mishra P and Samanta M (2007). Genotyping of Edwardsiella tarda isolated from freshwater fish culture system. Comparative Immunology, Microbiology and Infectious Diseases 30 33–40.
Algammal AM, Mabrok M, Ezzat M, Alfifi KJ, Esawy AM, Elmasry N, et al. (2022) Prevalence, antimicrobial resistance (AMR) pattern, virulence determinant and AMR genes of emerging multi-drug resistant Edwardsiella tarda in Nile tilapia and African catfish. Aquaculture. 548, 737643
Buján N, Toranzo AE, Magariños B. (2018). Edwardsiella piscicida: A significant bacterial pathogen of cultured fish. Disease Aquatic Organisms 131(1):59–71.
Chang CI, Wu CC, Cheng TC, Tsai JM, Lin KJ (2009). Multiplex nested‐polymerase chain reaction for the simultaneous detection of Aeromonas hydrophila, Edwardsiella tarda, Photobacterium damselae and Streptococcus iniae, four important fish pathogens in subtropical Asia. Aquaculture Research. 40: 1182-1190.
El Seedy FR., Radwan IA., Abd Rl-Galil MA. and Sayed HH. (2015). ''Phenotypic and Genotypic characterization of Edwardsiella tarda isolate from Oreochromis niloticus and Clarias gariepinus at Sohag Governoate. Journal of American Science, 11(11) 68-75.
Elgendy MY, Sherif AH, Kenawy AM, Abdelsalam M. (2022) Phenotypic and molecular characterization of the causative agents of edwardsiellosis causing Nile tilapia (Oreochromis niloticus) summer mortalities. Microbial Pathogenesis 169:105620.
Essa MAA., Mortada MA, Abd El-Galil MAA. and Korni FMM. (2012). Diagnosis and safe prevention of edwardsiellosis in Oreochromis niloticus. The Global Journal of Fisheries and Aqua. Res, 5, 147-159.
Ewing, WH, McWhorter AC, Escobar MR, and Lubin AH. (1965). Edwardsiella a new genus of Enterobacteriaceae based on a new species, E. tarda. International Bulletin of Bacterial Nomenclature and Taxonomy 15:33-38.
Griffin, MJ, Reichley, SR, Baumgartner, WA, Aarattuthodiyil, S, Ware, C, Steadman, JM, Lewis, M, Gaunt, PS, Khoo, LH, & Wise, D J. (2019). Emergence of Edwardsiella piscicida in farmed Channel♀, Ictalurus punctatus× Blue♂, Ictalurus furcatus, hybrid catfish cultured in Mississippi. Journal of the World Aquaculture Society, 50, 420–432.
Jiang J, Fan Y, Zhang S, Wang Q, Zhang Y, Liu Q, Shao S. (2022). Rapid on-the-spot detection of Edwardsiella piscicida using recombinase polymerase amplification with lateral flow, Aquaculture Reports, 22: 100945,
Korni F. M.M., Abo El-Ela FI., Moawad UK., Mahmoud RK., Gadelhak YM (2021). Prevention of Edwardsiellosis in Clarias gariepinus using ginger and its nanoparticles with a reference to histopathological alterations. Aquaculture 539, 736603
Leung KY., Wang Q., Yang Z., Siame BA. (2019). Edwardsiella piscicida: A versatile emerging pathogen of fish. Virulence, 10(1): 555–567.
López‐Porras, A, Griffin, MJ, Armwood, AR. et al. (2021). Genetic variability of Edwardsiella piscicida isolates from Mississippi catfish aquaculture with an assessment of virulence in channel and channel× blue hybrid catfish. Journal of Fish Diseases, 44, 1725–1751.
Matsuyama T, Kamaishi T, Ooseko N, Kurohara K, Iida T (2005). Pathogenicity of motile and non-motile Edwardsiella tarda to some marine fish. Fish Pathology 40: 133-136.
Miyazaki, T., and S. Egusa. (1976a). Histopathological studies of Edwardsiellosis of Japanese eel. I. Natural infection-suppurative interstitial nephritis. Fish Pathology 11:33-43.
Miyazaki, T., and S. Egusa. (1976b). Histopathological studies of Edwardsiellosis of Japanese eel. II. Suppurative hepatitis. Fish Pathology 11:67-75.
Noor El Deen AI, El-Gohary MS, Abdou MS and El-Gamal AM. 2017. Molecular characterization of Edwardsiella tarda bacteria causing severe mortalities in cultured Oreochromis niloticus fish with treatment trials. International Journal of Current Research 9: 50962-50969.
Ootsubo M, Shimizu T, Tanaka R, Sawabe T, Tajima K, Yoshimizu M, Ezura Y, Ezaki T and Oyaizu H (2002). Oligonucleotide probe for detecting Enterobacteriaceae by in situ hybridization; Journal of Applied Microbiology 93 60–68.
Park S, Wakabayashi H, Watanabe Y: Serotype and virulence of Edwardsiella tarda isolated from eel and their environment. Fish Pathology, 1983, 18: 85-89.
Park SB, Aoki T, Jung TS. (2012) Pathogenesis of and strategies for preventing Edwardsiella tarda infection in fish. Vet Res, 43(1):67.
Schlenker C. and Surawicz C.M. (2009). Emerging infections of the gastrointestinal tract. Best Practice & Research Clinical Gastroenterology, 23; 89-99.
Shao J, Guo Q, Hu R, et al. (2018). Comparative genomic insights into the taxonomy of Edwardsiella tarda isolated from different hosts: marine, freshwater and migratory fish. Aquaculture Research 49(1):197–204.
Swain P, Nayak SK (2003). Comparative sensitivity of different serological tests for seromonitoring and surveillance of Edwardsiella tarda infection of Indian major carps. Fish and Shellfish Immunology, 15: 333-340.
Walton DT, Abbott SL, Janda JM (1993). Sucrose-positive Edwardsiella tarda mimicking a biogroup 1 strain isolated from a patient with cholelithiasis. Journal of Clinical Microbiology, 31: 155-156.
Wang IK, Kuo HL, Chen YM, et al. (2005) Extraintestinal manifestations of Edwardsiella tarda infection. International Journal of clinical Practices 59(8):917–921.
Yu L, Yuan L, Feng H and Li SF (2004). Determination of the bacterial pathogen Edwardsiella tarda in fish species by capillary electrophoresis with blue light-emitting diode-induced fluorescence; Electrophoresis, 25: 3139–3144