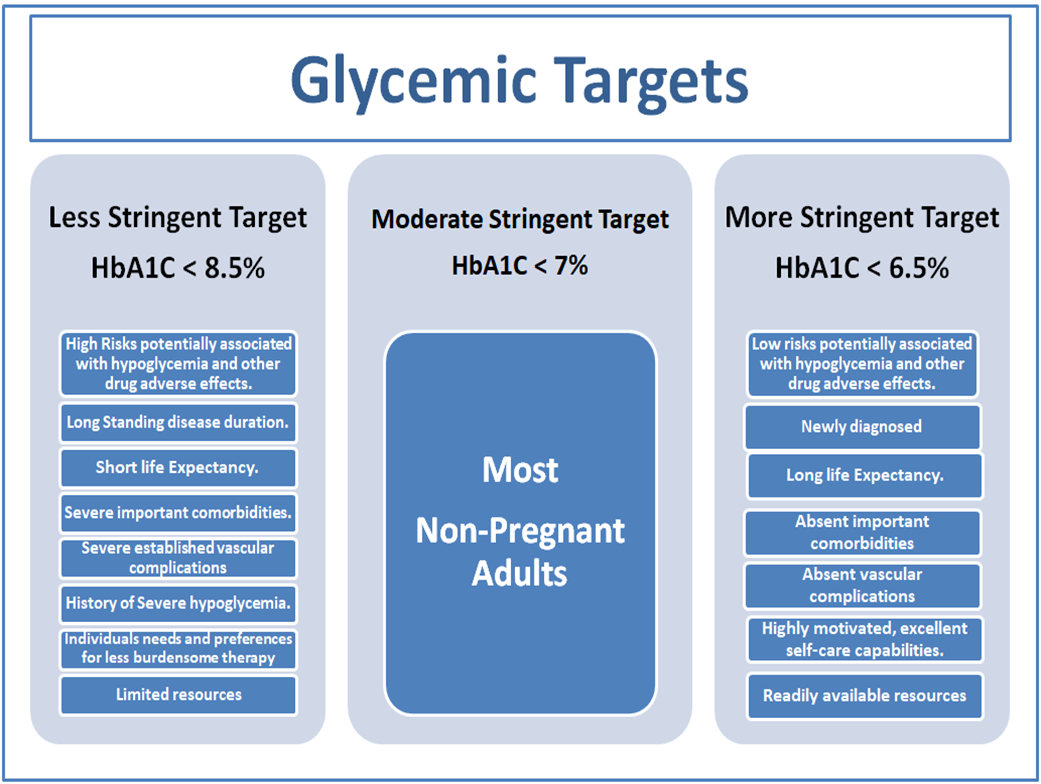
Glycemic Targets
| Site: | EHC | Egyptian Health Council |
| Course: | Diabetes and Endocrinology Guidelines |
| Book: | Glycemic Targets |
| Printed by: | Guest user |
| Date: | Monday, 23 December 2024, 10:00 PM |
Description
"last update: 30 April 2024"
- Committee
Acknowledgement:
We would like to acknowledge the Diabetes and Endocrinology Scientific Committee for developing these guidelines.
Chair of the GDG: Mohamed Hesham El Hefnawy, National Institute of Diabetes and Endocrinology, Cairo
Members of the Guideline Development Group (GDG):
Athar Reda Ibrahim, National Institute of Diabetes and Endocrinology, Cairo
Amr Ali Mahfouz, National Institute of Diabetes and Endocrinology, Cairo
Ahmed Mohamed Abdelfattah Hamam, Military Hospitals, Cairo
Atef Bassyouni, National Institute of Diabetes and Endocrinology, Cairo
Elsayed Abdel Fattah Eid, Faculty of Medicine, Delta University for science and technology, Dakahlia
Fawzy A F Elmessallamy, Faculty of Medicine, Zagazig University, Sharqia
Mai Mohammed Salama, National Hepatology and Tropical Medicine Research Institute, Cairo
Mohamed Abdelhady Mohamed Mashahet, Faculty of Medicine, Fayoum University, Fayoum.
Mohamed Abdelmoniem Elmikawy, police hospitals, Cairo.
Randa Salam, Faculty of Medicine, Cairo University, Cairo.
Yara Muhammad Ahmad Eid, Faculty of Medicine, Ain Shams University, Cairo.
- Abbreviations
BGM
Blood Glucose Monitoring
CGM
Continuous Glucose Monitoring
GRADE
Grading of Recommendations Assessment, Development and Evaluation
HbA1c
Glycated hemoglobin A1C
RCT
Randomized controlled trial
TAR
Time above Range
TBR
Time below Range
TIR
Time in Range
- Glossary
HbA1c
Glycated haemoglobin by non-enzymatic attachment of glucose to haemoglobin. The concentration of HbA1c is the most commonly used measure of chronic glycaemia in clinical trials and diabetes management. It is considered to reflect the integrated mean glucose level over the previous 8–12 weeks.
TAR
% of readings and time that blood glucose level >180 mg/dL
TBR
% of readings and time blood glucose level < 70 mg/dL
TIR
% of readings and time blood glucose level 70 - 180 mg/dL
- Executive Summary
This guideline offers evidence-based recommendations on the targeted levels of blood glucose. The recommendations are intended to provide healthcare professionals with practical guidance on monitoring of blood glucose and improving health outcomes for people living with Diabetes.
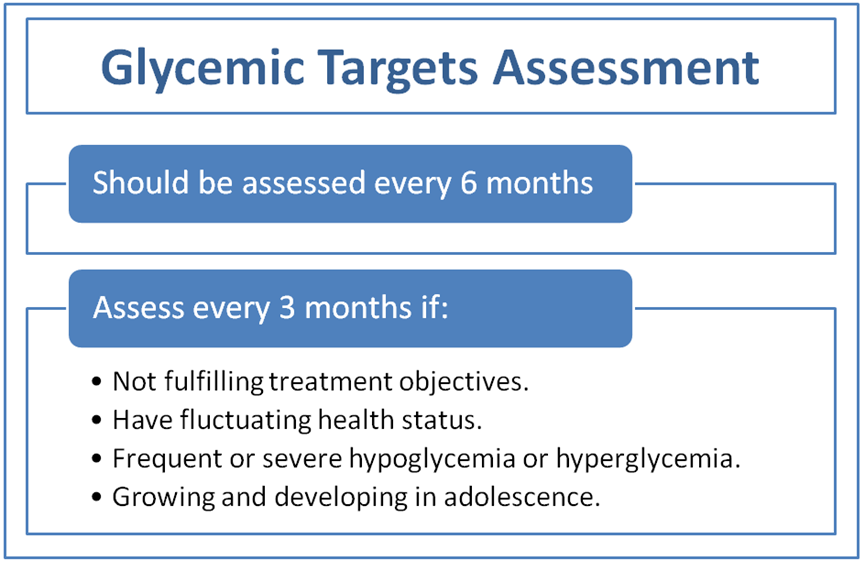
l Glycemic
status should be assessed at least twice a year using HbA1c and/or suitable
continuous glucose monitoring (CGM) parameters. Individuals who are not
fulfilling treatment objectives, have frequent or severe hypoglycemia or
hyperglycemia, have fluctuating health status, or are growing and developing
in adolescence should be assessed more regularly (every three months). (Good practice statement) l Glycemic
status should be assessed at least quarterly and as needed in people whose
therapy has recently changed and/or who are not achieving their glycemic
targets. (Good practice statement) l An HbA1c
target for many nonpregnant adults of <7% without significant hypoglycemia
is recommended. (strong recommendation) l
Time in range is associated with the risk of
microvascular complications and can be used for assessment of glycemic
control. Additionally, time below range and time above range are useful
parameters for the evaluation of the treatment plan. (Conditional recommendation). l
If using an ambulatory glucose profile/glucose
management indicator to assess glycemia, a parallel goal for many nonpregnant
adults is TIR >70% with time below range <4% and time <54 mg/dL <1%.
For those with frailty or at high risk of hypoglycemia, a goal of >50% TIR
with <1% time below range is recommended. (Conditional recommendation). l
On the basis of health care professional judgment
and patient preference, achievement of lower HbA1c levels than the goal of 7%
may be acceptable and even beneficial if it can be achieved safely without
significant hypoglycemia or other adverse effects of treatment. (Strong recommendation) l Less
stringent HbA1c targets (such as <8% may be appropriate for patients with
limited life expectancy or where the harms of treatment are greater than the
benefits. (Strong recommendation). l
Healthcare professionals should consider
deintensification of therapy if appropriate to reduce the risk of
hypoglycemia in patients with inappropriate stringent HbA1c targets. (Strong recommendation).
Recommendations
- Introduction
Glycemic targets and management should be individualized to the person rather than a one-size-fits-all strategy. To prevent both microvascular and macrovascular complications of diabetes, there is a strong need to overcome therapeutic inertia and treat patients based on individual needs. HbA1c test, continuous glucose monitoring (CGM) with time in range (TIR) and/or glucose management indicator (GMI), and blood glucose monitoring (BGM) are all used to assess glycemic control. HbA1c is the statistic used in clinical studies to demonstrate the advantages of better glycemic management. Individual glucose monitoring is a valuable tool for diabetes self-management, which includes meals, physical activity, and prescription adjustments, especially for insulin users. CGM provides an increasingly essential role in managing the effectiveness and safety of therapy in many patients with type 1 diabetes and certain people with type 2 diabetes. Individuals on various insulin treatment schemes might benefit from CGM by improving glucose control, reducing hypoglycemia, and increasing self-efficacy
- Scope and Purpose
The objectives of this guidelines is :
- To provide guidance for the proper glycemic targets for optimal glycemic control.
- To provide guidance on various methods for monitoring of glucose levels to be used by individuals with diabetes and health professionals to optimizing blood glucose level for better glucose control, reducing hypoglycemia, and increasing self-efficacy.
- Target Audience
This guideline targets; healthcare professionals, policy makers, national diabetes programme managers, as well as non-governmental organizations (NGOs) and other stakeholders to afford the most appropriate tools for individuals with diabetes.
Methodology:
A comprehensive search for guidelines was undertaken to identify the most relevant guidelines to consider for adaptation.
Inclusion/ exclusion criteria followed in the search and retrieval of guidelines to be adapted:
• Selecting only evidence-based guidelines (guideline must include a report on systematic literature searches and explicit links between individual recommendations and their supporting evidence)
• Selecting only national and/or international guidelines
• Specific range of dates for publication (using Guidelines published or updated in 2015 and later)
• Selecting peer reviewed publications only
• Selecting guidelines written in English language
• Excluding guidelines written by a single author, not on behalf of an organization to be valid and comprehensive, a guideline ideally requires multidisciplinary input
• Excluding guidelines published without references as the panel needs to know whether a thorough literature review was conducted and whether current evidence was used in the preparation of the recommendations
The following characteristics of the retrieved guidelines were summarized in:
• Developing organisation/authors
• Date of publication, posting, and release
• Country/language of publication
• Date of posting and/or release
• Dates of the search used by the source guideline developers
All retrieved Guidelines were screened and appraised using AGREE II instrument (www.agreetrust.org) by at least three members. The panel decided on a cut-off point or ranked the guidelines (any guideline scoring above 50% on the rigor dimension was retained). The GDG decided to adapt the American Diabetes Association – Standards of Care in Diabetes – 2024.
- Evidence assessment
According to WHO Handbook for Guidelines, we used the GRADE (Grading of
Recommendations, Assessment, Development and Evaluation) approach to assess the quality of a body of evidence, develop and report recommendations. GRADE methods are used by WHO because these represent internationally agreed standards for making transparent recommendations. Detailed GRADE information is available on the following sites:
• GRADE working group: http://www.gradeworkingroup.org
• GRADE online training modules: http://cebgrade.mcmaster.ca/
• GRADE profile software: http://ims.cochrane.org/revman/gradepro
Table 1 Quality and Significance of the four levels of evidence in GRADE:
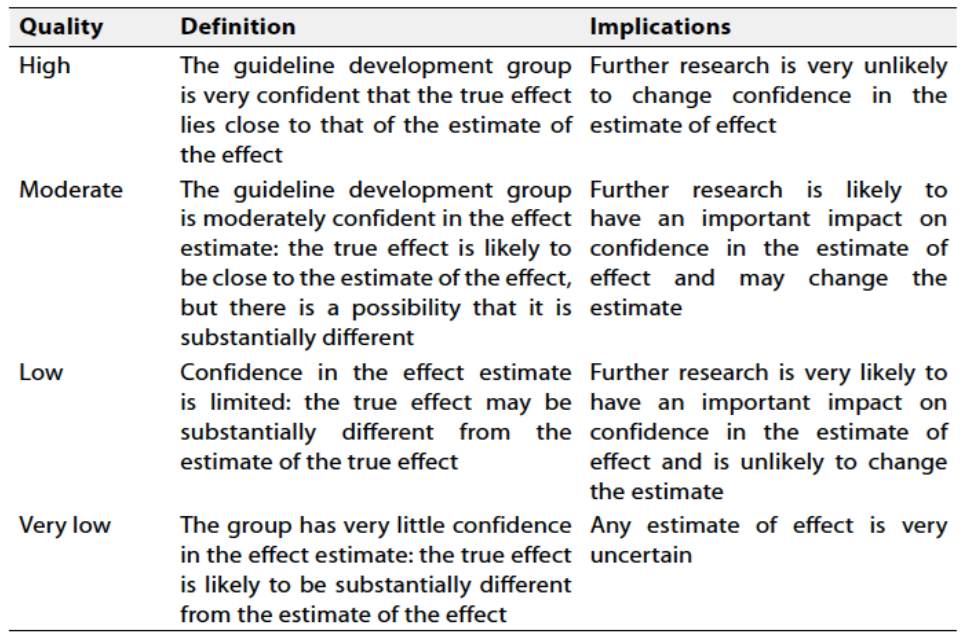
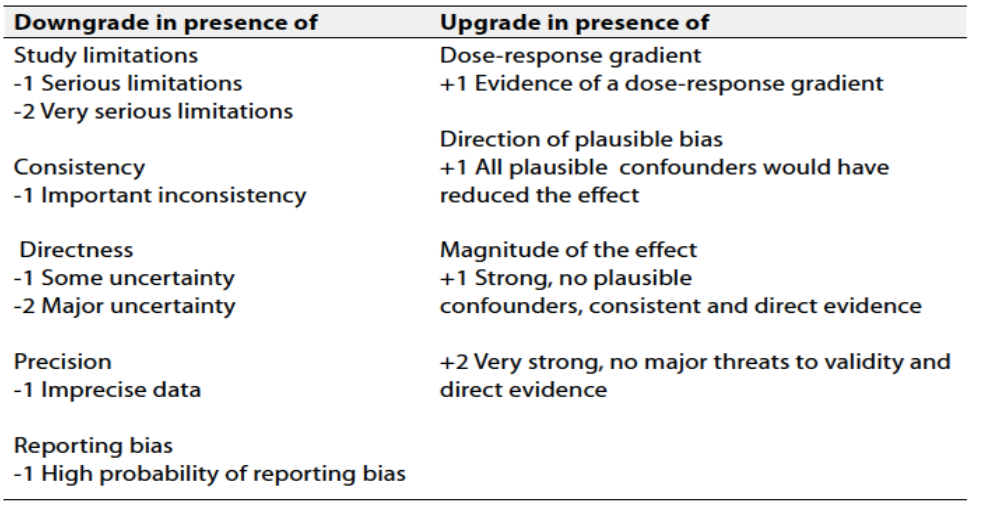
Table 2 Factors that determine How to upgrade or downgrade the quality of evidence
The strength of the recommendation
The strength of a recommendation communicates the importance of adherence to the recommendation.
➡️Strong recommendations
With strong recommendations, the guideline communicates the message that the desirable effects of adherence to the recommendation outweigh the undesirable effects. This means that in most situations the recommendation can be adopted as policy.
➡️Conditional recommendations
These are made when there is greater uncertainty about the four factors above or if local adaptation has to account for a greater variety in values and preferences, or when resource use makes the intervention suitable for some, but not for other locations. This means that there is a need for substantial debate and involvement of stakeholders before this recommendation can be adopted as policy.
➡️When not to make recommendations
When there is lack of evidence on the effectiveness of an intervention, it may be appropriate not to make a recommendation.
- Recommendations
Recommendation
l Glycemic status should be assessed at least twice a year using HbA1c. Individuals who are not fulfilling treatment objectives, have frequent or severe hypoglycemia or hyperglycemia, have fluctuating health status, or are growing and developing in adolescence should be assessed more regularly (every three months).
(Good practice statement, low certainty evidence)
l Glycemic status should be assessed at least quarterly and as needed in people whose therapy has recently changed and/or who are not achieving their glycemic targets.
(Good practice statement, low certainty evidence)
l An HbA1c goal for nonpregnant adults of <7% without significant hypoglycemia is appropriate.
(strong recommendation, high certainty evidence )
➡️Remarks
It is reasonable to check postprandial glucose in individuals who have premeal glucose values within target but HbA1c values above target. In addition, when intensifying insulin therapy, measuring postprandial plasma glucose 1–2 h after the start of a meal (using BGM or CGM) and using treatments aimed at reducing postprandial plasma glucose values to <180 mg/dL (10.0 mmol/L) may help to lower HbA1c.
➡️Summary of evidence
The Diabetes Control and Complications Trial (DCCT)[i], a prospective randomized controlled trial of intensive (mean HbA1c about 7% [53 mmol/mol]) versus standard (mean HbA1c about 9% [75 mmol/mol]) glycemic control in people with type 1 diabetes, showed definitively that better glycemic control is associated with 50–76% reductions in rates of development and progression of microvascular (retinopathy, neuropathy, and diabetic kidney disease) complications. Follow-up of the DCCT cohorts in the Epidemiology of Diabetes Interventions and Complications (EDIC) study[ii]’[iii] demonstrated persistence of these microvascular benefits over two decades despite the fact that the glycemic separation between the treatment groups diminished and disappeared during follow-up. The Kumamoto Study[iv] and UK Prospective Diabetes Study (UKPDS)[v]’[vi] confirmed that intensive glycemic control significantly decreased rates of microvascular complications in people with short-duration type 2 diabetes. Long-term follow-up of the UKPDS cohorts showed enduring effects of early glycemic control on most microvascular complications[vii].
[i] Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977– 986.
[ii] Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group; Lachin JM, White NH, Hainsworth DP, et al. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes 2015;64:631–642.
[iii] Diabetes Control and Complications Trial/ Epidemiology of Diabetes Interventions and Complications Research Group; Lachin JM, Genuth S, Cleary P, Davis MD, Nathan DM. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 2000;342:381–389.
[iv] Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–117.
[v] UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865.
[vi] UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853.
[vii] Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589.
➡️Rationale for the recommendation
Achieving HbA1c targets of <7% has been shown to reduce microvascular complications of type 1 and type 2 diabetes when instituted early in the course of disease[i]’[ii].
[i] Laiteerapong N, Ham SA, Gao Y, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the Diabetes & Aging Study). Diabetes Care 2019;42: 416–426.
[ii] Lind M, Pivodic A, Svensson AM, Olafsdottir AF, Wedel H, Ludvigsson J. HbA1c level as a risk factor for retinopathy and nephropathy in children and adults with type 1 diabetes: Swedish population based cohort study. BMJ 2019;366: l4894.
➡️Recommendation:
l Time in range is associated with the risk of microvascular complications and can be used for assessment of glycemic control. Additionally, time below range and time above range are useful parameters for the evaluation of the treatment plan.
(Conditional recommendation, moderate certainty evidence).
➡️Remarks
Time in Range (TIR) is a useful metric of glycemic control and glucose patterns, and it correlates well with HbA1c in most studies. TIR can be used for assessment of glycemic control. Additionally, time below range (<70 and <54 mg/dL [3.9 and 3.0 mmol/L]) and time above range (>180 mg/dL [10.0 mmol/L]) are useful parameters for insulin dose adjustments and reevaluation of the treatment plan.The international consensus on TIR provides guidance on standardized CGM metrics (Table 3) and considerations for clinical interpretation and care[i].
Table 3. Standardized CGM metrics for clinical care
[i] Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603
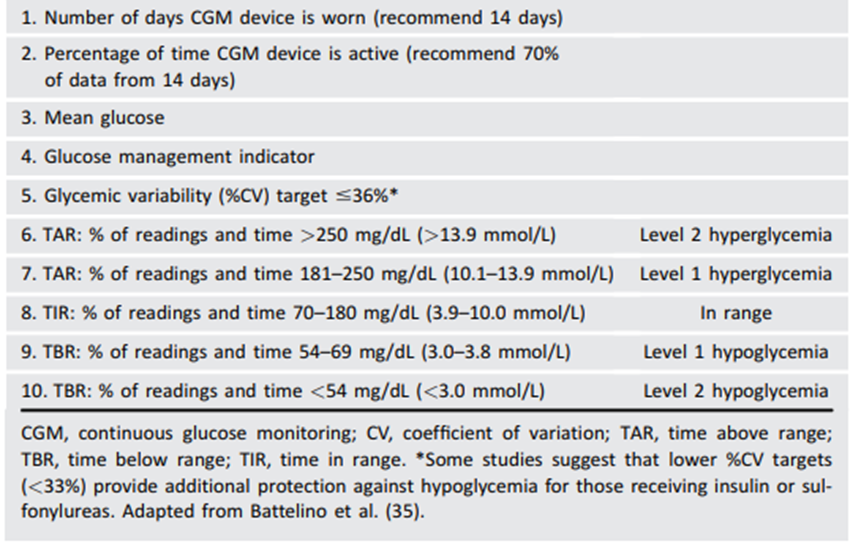
To make these metrics more actionable, standardized reports with visual cues, such as the ambulatory glucose profile (Fig 1), are recommended10 and may help the patient and the health care professional better interpret the data to guide treatment decisions[i]’[ii]. BGM and CGM can be useful to guide medical nutrition therapy and physical activity, prevent hypoglycemia, and aid medication management. While HbA1c is currently the primary measure to guide glucose management and a valuable risk marker for developing diabetes complications, the CGM metrics TIR (with time below range and time above range) and GMI provide the insights for a more personalized diabetes management plan. The incorporation of these metrics into clinical practice is in evolution, and remote access to these data can be critical for
[i] Advani A. Positioning time in range in diabetes management. Diabetologia 2020;63:242–252
[ii] Kr€oger J, Reichel A, Siegmund T, Ziegler R. Clinical recommendations for the use of the ambulatory glucose profile in diabetes care. J Diabetes Sci Technol 2020;14:586–594
telehealth. A rapid optimization and harmonization of CGM terminology and remote access is occurring to meet patient and health care professional needs[i]’[ii]’[iii]. The patient’s specific needs and goals should dictate BGM frequency and timing and consideration of CGM use.
Table 4 Summary of recommendations for many nonpregnant adults with diabetes
[i]Tchero H, Kangambega P, Briatte C, BrunetHoudard S, Retali GR, Rusch E. Clinical effectiveness of telemedicine in diabetes mellitus: a meta-analysis of 42 randomized controlled trials. Telemed J E Health 2019;25:569–583
[ii] Salabelle C, Ly Sall K, Eroukhmanoff J, et al. COVID-19 pandemic lockdown in young people with type 1 diabetes: positive results of an unprecedented challenge for patients through telemedicine and change in use of continuous glucose monitoring. Prim Care Diabetes 2021;15:884–886.
[iii] Prabhu Navis J, Leelarathna L, Mubita W, et al. Impact of COVID-19 lockdown on flash and realtime glucose sensor users with type 1 diabetes in England. Acta Diabetol 2021;58:231–237.

➡️Summary of evidence
Data support the premise that increased TIR correlates with the risk of complications. Many studies supporting this assertion; they include cross-sectional data and cohort studies[i]’[ii]’[iii] demonstrating TIR as an acceptable end point for clinical trials moving forward and that it can be used for assessment of glycemic control. Additionally, time below range (<70 and <54 mg/dL [3.9 and 3.0 mmol/L]) and time above range (>180 mg/dL [10.0 mmol/L]) are useful parameters for insulin dose adjustments and reevaluation of the treatment plan.
➡️Rationale for the recommendation
Time in range (TIR) is a useful metric of glycemic control and glucose patterns, and it correlates well with HbA1c in most studies11’[iv]’[v]’12’[vi]’[vii].
➡️Recommendation:
l If using ambulatory glucose profile/glucose management indicator to assess glycemia, a parallel goal for many nonpregnant adults is time in range of >70% with time below range <4% and time <54 mg/dL <1%. For those with frailty or at high risk of hypoglycemia, a target of >50% time in range with <1% time below range is recommended. (Table 3) (Conditional recommendation, moderate certainty evidence).
[i] Mayeda L, Katz R, Ahmad I, et al. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res Care 2020;8:e000991
[ii] Yoo JH, Choi MS, Ahn J, et al. Association between continuous glucose monitoring-derived time in range, other core metrics, and albuminuria in type 2 diabetes. Diabetes Technol Ther 2020; 22:768–776
[iii] Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther 2020;22: 72–78
[iv] Avari P, Uduku C, George D, Herrero P, Reddy M, Oliver N. Differences for percentage times in glycemic range between continuous glucose monitoring and capillary blood glucose monitoring in adults with type 1 diabetes: analysis of the REPLACE-BG dataset. Diabetes Technol Ther 2020; 22:222–227
[v] Vigersky RA, McMahon C.The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther 2019;21: 81–85
[vi] Livingstone R, Boyle JG, Petrie JR. How tightly controlled do fluctuations in blood glucose levels need to be to reduce the risk of developing complications in people with type 1 diabetes? Diabet Med 2020;37:513–521
[vii] Messer LH, Berget C, Vigers T, et al. Real world hybrid closed-loop discontinuation: Predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes 2020;21:319–327
➡️Remarks
With the advent of new technology, CGM has evolved rapidly in both accuracy and affordability. As such, many patients have these data available to assist with self-management and their health care professionals’ assessment of glycemic status. Reports can be generated from CGM that will allow the health care professional and person with diabetes to determine TIR, calculate GMI, and assess hypoglycemia, hyperglycemia, and glycemic variability. As discussed in a recent consensus document, a report formatted as shown in Fig. 6.1 can be generated10. Published data from two retrospective studies suggest a strong correlation between TIR and HbA1c, with a goal of 70% TIR aligning with an HbA1c of 7% 20’[i]. Note the goals of therapy next to each metric in Fig. 6.1 (e.g., low, <4%; very low, <1%) as values to guide changes in therapy.
➡️Summary of evidence
CGM is rapidly improving diabetes management. As stated in the recommendations, time in range (TIR) is a useful metric of glycemic control and glucose patterns, and it correlates well with HbA1c in most studies11’[ii]’[iii]’12’[iv]’[v]. New data support the premise that increased TIR correlates with the risk of complications. The studies supporting this assertion include crosssectional data and cohort studies[vi]’[vii]’[viii]. Demonstrating TIR as an acceptable end point for clinical trials moving forward and that it can be used for assessment of glycemic control. Additionally, time below range (<70 and <54 mg/dL [3.9 and 3.0 mmol/L]) and time above range (>180 mg/dL [10.0 mmol/L]) are useful parameters for insulin dose adjustments and reevaluation of the treatment plan.
Rationale for the recommendation
➡️Recommendation
l On the basis of health care professional judgment and patient preference, achievement of lower HbA1c levels than the goal of 7% may be acceptable and even beneficial if it can be achieved safely without significant hypoglycemia or other adverse effects of treatment. (Strong recommendation, moderate certainty evidence).
➡️Remarks
based on clinician judgment and patient preferences, select patients, especially those with little comorbidity and a long life expectancy, may benefit from adopting more intensive glycemic targets if they can achieve them safely and without hypoglycemia or significant therapeutic burden.
[i] Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol 2019; 13:614–626
[ii] Avari P, Uduku C, George D, Herrero P, Reddy M, Oliver N. Differences for percentage times in glycemic range between continuous glucose monitoring and capillary blood glucose monitoring in adults with type 1 diabetes: analysis of the REPLACE-BG dataset. Diabetes Technol Ther 2020; 22:222–227
[iii] Vigersky RA, McMahon C.The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther 2019;21: 81–85
[iv] Livingstone R, Boyle JG, Petrie JR. How tightly controlled do fluctuations in blood glucose levels need to be to reduce the risk of developing complications in people with type 1 diabetes? Diabet Med 2020;37:513–521
[v] Messer LH, Berget C, Vigers T, et al. Real world hybrid closed-loop discontinuation: Predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes 2020;21:319–327
[vi] Mayeda L, Katz R, Ahmad I, et al. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res Care 2020;8:e000991
[vii] Yoo JH, Choi MS, Ahn J, et al. Association between continuous glucose monitoring-derived time in range, other core metrics, and albuminuria in type 2 diabetes. Diabetes Technol Ther 2020; 22:768–776
[viii] Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther 2020;22: 72–78
➡️Summary of evidence
CGM is rapidly improving diabetes management. As stated in the recommendations, time in range (TIR) is a useful metric of glycemic control and glucose patterns, and it correlates well with HbA1c in most studies11’[i]’[ii]’12’[iii]’[iv]. New data support the premise that increased TIR correlates with the risk of complications. The studies supporting this assertion include crosssectional data and cohort studies[v]’[vi]’[vii]. Demonstrating TIR as an acceptable end point for clinical trials moving forward and that it can be used for assessment of glycemic control. Additionally, time below range (<70 and <54 mg/dL [3.9 and 3.0 mmol/L]) and time above range (>180 mg/dL [10.0 mmol/L]) are useful parameters for insulin dose adjustments and reevaluation of the treatment plan.
Rationale for the recommendation
➡️Recommendation
l On the basis of health care professional judgment and patient preference, achievement of lower HbA1c levels than the goal of 7% may be acceptable and even beneficial if it can be achieved safely without significant hypoglycemia or other adverse effects of treatment. (Strong recommendation, moderate certainty evidence).
➡️Remarks
based on clinician judgment and patient preferences, select patients, especially those with little comorbidity and a long life expectancy, may benefit from adopting more intensive glycemic targets if they can achieve them safely and without hypoglycemia or significant therapeutic burden.
➡️Summary of evidence
Findings from the DCCT1. and UKPDS[viii] studies demonstrate a curvilinear relationship between HbA1c and microvascular complications. Such analyses suggest that, on a population level, the greatest number of complications will be averted by taking patients from very poor control to fair/good control. These analyses also suggest that further lowering of HbA1c from 7 to 6% (53 mmol/mol to 42 mmol/mol) is associated with further reduction in the risk of microvascular complications, although the absolute risk reductions become much smaller. The implication of these findings is that there is no need to deintensify therapy for an individual with an HbA1c between 6 and 7% in the setting of low hypoglycemia risk with a long life expectancy. There are now newer agents that do not cause hypoglycemia, making it possible to maintain glucose control without the risk of hypoglycemia. Given the substantially increased risk of hypoglycemia in type 1 diabetes and with polypharmacy in type 2 diabetes, the risks of lower glycemic targets may outweigh the potential benefits on microvascular complications. Three landmark trials (Action to Control Cardiovascular Risk in Diabetes [ACCORD], Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation [ADVANCE], and Veterans Affairs Diabetes Trial [VADT]) were conducted to test the effects of near normalization of blood glucose on cardiovascular outcomes in individuals with long-standing type 2 diabetes and either known cardiovascular disease (CVD) or high cardiovascular risk. These trials showed that lower A1C levels were associated with reduced onset or progression of some microvascular complication[ix]’[x]’[xi].
The concerning mortality findings in the ACCORD trial discussed below and the relatively intense efforts required to achieve near euglycemia should also be considered when setting glycemic targets for individuals with longstanding diabetes, such as those populations studied in ACCORD, ADVANCE, and VADT. Findings from these studies suggest caution is needed in treating diabetes to near-normal HbA1c goals in people with long-standing type 2 diabetes with or at significant risk of CVD.
These landmark studies need to be considered with an important caveat; glucagon-like peptide 1 (GLP-1) receptor agonists and sodium–glucose cotransporter 2 (SGLT2) inhibitors were not approved at the time of these trials. As such, these agents with established cardiovascular and renal benefits appear to be safe and beneficial in this group of individuals at high risk for cardiorenal complications. Randomized clinical trials examining these agents for cardiovascular safety were not designed to test higher versus lower HbA1c; therefore, beyond post hoc analysis of these trials, we do not have evidence that it is the glucose lowering by these agents that confers the CVD and renal benefit[xii]. As such, based on clinician judgment and patient preferences, select patients, especially those with little comorbidity and a long life expectancy, may benefit from adopting more intensive glycemic targets if they can achieve them safely and without hypoglycemia or significant therapeutic burden.There is evidence for a cardiovascular benefit of intensive glycemic control after long-term followup of cohorts treated early in the course of type 1 diabetes. In the DCCT, there was a trend toward lower risk of CVD events with intensive control. In the 9-year post-DCCT follow-up of the EDIC cohort, participants previously randomized to the intensive arm had a significant 57% reduction in the risk of nonfatal myocardial infarction (MI), stroke, or cardiovascular death compared with those previously randomized to the standard arm[xiii]. The benefit of intensive glycemic control in this cohort with type 1 diabetes has been shown to persist for several decades[xiv] and to be associated with a modest reduction in all-cause mortality[xv]. Cardiovascular Disease and Type 2 Diabetes In type 2 diabetes, there is evidence that more intensive treatment of glycemia in newly diagnosed patients may reduce long-term CVD rates. In addition, data from the Swedish National Diabetes Registry38 and the Joint Asia Diabetes Evaluation (JADE) demonstrate greater proportions of people with diabetes being diagnosed at <40 years of age and a demonstrably increased burden of heart disease and years of life lost in people diagnosed at a younger age[xvi]’[xvii]’[xviii]’[xix]. Thus, to prevent both microvascular and macrovascular complications of diabetes, there is a major call to overcome therapeutic inertia and treat to target for an individual patient42’[xx]. During the UKPDS, there was a 16% reduction in CVD events (combined fatal or nonfatal MI and sudden death) in the intensive glycemic control arm that did not reach statistical significance (P = 0.052), and there was no suggestion of benefit on other CVD outcomes (e.g., stroke). Similar to the DCCT/EDIC, after 10 years of observational followup, those originally randomized to intensive glycemic control had significant long-term reductions in MI (15% with sulfonylurea or insulin as initial pharmacotherapy, 33% with metformin as initial pharmacotherapy) and in all-cause mortality (13% and 27%, respectively)7. ACCORD, ADVANCE, and VADT suggested no significant reduction in CVD outcomes with intensive glycemic control in participants followed for shorter durations (3.5–5.6 years) and who had more advanced type 2 diabetes and CVD risk than the UKPDS participants. All three trials were conducted in relatively older participants with a longer known duration of diabetes (mean duration 8–11 years) and either CVD or multiple cardiovascular risk factors. The target HbA1c among intensive-control participants was <6% (42 mmol/mol) in ACCORD, <6.5% (48 mmol/mol) in ADVANCE, and a 1.5% reduction in HbA1c compared with control participants in VADT, with achieved HbA1c of 6.4% vs. 7.5% (46 mmol/mol vs. 58 mmol/mol) in ACCORD, 6.5% vs. 7.3% (48 mmol/mol vs. 56 mmol/mol) in ADVANCE, and 6.9% vs. 8.4% (52 mmol/mol vs. 68 mmol/mol) in VADT. Details of these studies are reviewed extensively in the joint ADA position statement “Intensive Glycemic Control and the Prevention of Cardiovascular Events: Implications of the ACCORD, ADVANCE, and VA Diabetes Trials”43.
➡️Rationale for the recommendation
Achieving HbA1c targets of <7% (53 mmol/mol) has been shown to reduce microvascular complications of type 1 and type 2 diabetes when instituted early in the course of disease8’9.
➡️Recommendation
l Less stringent HbA1c goals (such as <8% may be appropriate for patients with limited life expectancy or where the harms of treatment are greater than the benefits.
(Strong recommendation, moderate certainty evidence)
l Healthcare professionals should consider deintensification of therapy if appropriate to reduce the risk of hypoglycemia in patients with inappropriate stringent HbA1c targets.
(Strong recommendation, moderate certainty evidence).
➡️Remarks
Less stringent targets (HbA1c up to 8%) may be recommended if the patient’s life expectancy is such that the benefits of an intensive goal may not be realized, or if the risks and burdens outweigh the potential benefits. Severe or frequent hypoglycemia is an absolute indication for the modification of treatment plans, including setting higher glycemic goals.
➡️Summary of evidence
The glycemic control comparison in ACCORD was halted early due to an increased mortality rate in the intensive compared with the standard treatment arm (1.41% vs. 1.14% per year; hazard ratio 1.22 [95% CI 1.01–1.46]), with a similar increase in cardiovascular deaths. Analysis of the ACCORD data did not identify a clear explanation for the excess mortality in the intensive treatment arm[xxi]. Mortality findings in ACCORD[xxii] and subgroup analyses of VADT[xxiii] suggest that the potential risks of intensive glycemic control may outweigh its benefits in higher-risk individuals. In all three trials, severe hypoglycemia was significantly more likely in participants who were randomly assigned to the intensive glycemic control arm. Individuals with a long duration of diabetes, a known history of hypoglycemia, advanced atherosclerosis, or advanced age/frailty may benefit from less aggressive targets[xxiv]’[xxv]. Both DCCT/ EDIC and UKPDS demonstrated metabolic memory, or a legacy effect, in which a finite period of intensive control yielded benefits that extended for decades after that control ended. Thus, a finite period of intensive control to near-normal HbA1c may yield enduring benefits even if control is subsequently deintensified as patient characteristics change. Over time, comorbidities may emerge, decreasing life expectancy and thereby decreasing the potential to reap benefits from intensive control. Also, with longer disease duration, diabetes may become more difficult to control, with increasing risks and burdens of therapy. Thus, HbA1c targets should be reevaluated over time to balance the risks and benefits as patient factors change.
➡️Rationale for the recommendation
Severe hypoglycemia is a potent marker of high absolute risk of cardiovascular events and mortality[xxvi]. Therefore, health care professionals should be vigilant in preventing hypoglycemia and should not aggressively attempt to achieve near-normal HbA1c levels in people in whom such targets cannot be safely and reasonably achieved.
[i] Avari P, Uduku C, George D, Herrero P, Reddy M, Oliver N. Differences for percentage times in glycemic range between continuous glucose monitoring and capillary blood glucose monitoring in adults with type 1 diabetes: analysis of the REPLACE-BG dataset. Diabetes Technol Ther 2020; 22:222–227
[ii] Vigersky RA, McMahon C.The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther 2019;21: 81–85
[iii] Livingstone R, Boyle JG, Petrie JR. How tightly controlled do fluctuations in blood glucose levels need to be to reduce the risk of developing complications in people with type 1 diabetes? Diabet Med 2020;37:513–521
[iv] Messer LH, Berget C, Vigers T, et al. Real world hybrid closed-loop discontinuation: Predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes 2020;21:319–327
[v] Mayeda L, Katz R, Ahmad I, et al. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res Care 2020;8:e000991
[vi] Yoo JH, Choi MS, Ahn J, et al. Association between continuous glucose monitoring-derived time in range, other core metrics, and albuminuria in type 2 diabetes. Diabetes Technol Ther 2020; 22:768–776
[vii] Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther 2020;22: 72–78
[viii] Adler AI, Stratton IM, Neil HAW, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000;321:412–419
[ix] Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139
[x] Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358: 2560–2572
[xi] Ismail-Beigi F, Craven T, Banerji MA, et al.; ACCORD trial group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–430
[xii] Buse JB, Bain SC, Mann JFE, et al.; LEADER Trial Investigators. Cardiovascular risk reduction with liraglutide: an exploratory mediation analysis of the LEADER trial. Diabetes Care 2020;43: 1546–15
[xiii] Nathan DM, Cleary PA, Backlund JYC, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653
[xiv] Nathan DM, Zinman B, Cleary PA, et al.; Diabetes Control and Complications Trial/ Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983-2005). Arch Intern Med 2009;169:1307–1316
[xv] Emerging Risk Factors Collaboration; Di Angelantonio E, Kaptoge S, Wormser D, et al. Association of cardiometabolic multimorbidity with mortality. JAMA 2015;314:52–60
[xvi] Yeung RO, Zhang Y, Luk A, et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a crosssectional study of a prospective cohort. Lancet Diabetes Endocrinol 2014;2:935–943
[xvii] Sattar N, Rawshani A, Franzen S, et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation 2019;139:2228–2237
[xviii] Zabala A, Darsalia V, Holzmann MJ, et al. Risk of first stroke in people with type 2 diabetes and its relation to glycaemic control: a nationwide observational study. Diabetes Obes Metab 2020; 22:182–190
[xix] Zoungas S, Woodward M, Li Q, et al.; ADVANCE Collaborative group. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia 2014;57: 2465–2474
[xx] Skyler JS, Bergenstal R, Bonow RO, et al.; American Diabetes Association; American College of Cardiology Foundation; American Heart Association. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009;32:187–192
[xxi] Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358: 2545–2559
[xxii] Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358: 2545–2559
[xxiii] Duckworth WC, Abraira C, Moritz TE, et al.; Investigators of the VADT. The duration of diabetes affects the response to intensive glucose control in type 2 subjects: the VA Diabetes Trial. J Diabetes Complications 2011;25:355–361
[xxiv] Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356–362
[xxv] Vijan S, Sussman JB, Yudkin JS, Hayward RA. Effect of patients’ risks and preferences on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med 2014;174: 1227–1234
[xxvi] Lee AK, Warren B, Lee CJ, et al. The association of severe hypoglycemia with incident cardiovascular events and mortality in adults with type 2 diabetes. Diabetes Care 2018;41:104–111
- Implementation considerations
Several barriers may hinder the effective implementation and scale-up of the recommendations in this guideline. These factors may be related to the behaviours of patients (or families), the behavior of healthcare professionals, the organization of care, health service delivery or financial arrangements.
Obstacles to effective implementation include:
▪️ Patient engagement
▪️ Collaboration; person centered, team based collaboration between clinician, dietitian, pharmacist and others involved in care delivery
▪️ Behavior changes: information, guidance and support delivered easily and consistently can help assess sustained behavioral changes.
➡️Research needs
▪️ Clinical trials to evaluate the use of other biomarkers such as Fructosamine or glycated albumin to monitor glycemic status in people with diabetes who have conditions where the interpretation of HbA1c cannot be measured.
➡️Monitoring and evaluating the impact of the guideline
Assessment of effectiveness of diabetes services: Measure HbA1C at the recommended times.
➡️Updating of the guidelines:
These guidelines will be updated whenever there is new evidence.
Adapted from ADA “Standards of Care in Diabetes” 2024
- Reference
[1] Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977– 986.
[1] Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group; Lachin JM, White NH, Hainsworth DP, et al. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes 2015;64:631–642.
[1] Diabetes Control and Complications Trial/ Epidemiology of Diabetes Interventions and Complications Research Group; Lachin JM, Genuth S, Cleary P, Davis MD, Nathan DM. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 2000;342:381–389.
[1] Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–117.
[1] UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865.
[1] UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853.
[1] Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589.
[1] Laiteerapong N, Ham SA, Gao Y, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the Diabetes & Aging Study). Diabetes Care 2019;42: 416–426.
[1] Lind M, Pivodic A, Svensson AM, Olafsdottir AF, Wedel H, Ludvigsson J. HbA1c level as a risk factor for retinopathy and nephropathy in children and adults with type 1 diabetes: Swedish population based cohort study. BMJ 2019;366: l4894.
[1] Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603
[1] Advani A. Positioning time in range in diabetes management. Diabetologia 2020;63:242–252
[1] Kr€oger J, Reichel A, Siegmund T, Ziegler R. Clinical recommendations for the use of the ambulatory glucose profile in diabetes care. J Diabetes Sci Technol 2020;14:586–594
[1]Tchero H, Kangambega P, Briatte C, BrunetHoudard S, Retali GR, Rusch E. Clinical effectiveness of telemedicine in diabetes mellitus: a meta-analysis of 42 randomized controlled trials. Telemed J E Health 2019;25:569–583
[1] Salabelle C, Ly Sall K, Eroukhmanoff J, et al. COVID-19 pandemic lockdown in young people with type 1 diabetes: positive results of an unprecedented challenge for patients through telemedicine and change in use of continuous glucose monitoring. Prim Care Diabetes 2021;15:884–886.
[1] Prabhu Navis J, Leelarathna L, Mubita W, et al. Impact of COVID-19 lockdown on flash and realtime glucose sensor users with type 1 diabetes in England. Acta Diabetol 2021;58:231–237.
[1] Mayeda L, Katz R, Ahmad I, et al. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res Care 2020;8:e000991
[1] Yoo JH, Choi MS, Ahn J, et al. Association between continuous glucose monitoring-derived time in range, other core metrics, and albuminuria in type 2 diabetes. Diabetes Technol Ther 2020; 22:768–776
[1] Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther 2020;22: 72–78
[1] Avari P, Uduku C, George D, Herrero P, Reddy M, Oliver N. Differences for percentage times in glycemic range between continuous glucose monitoring and capillary blood glucose monitoring in adults with type 1 diabetes: analysis of the REPLACE-BG dataset. Diabetes Technol Ther 2020; 22:222–227
[1] Vigersky RA, McMahon C.The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther 2019;21: 81–85
[1] Livingstone R, Boyle JG, Petrie JR. How tightly controlled do fluctuations in blood glucose levels need to be to reduce the risk of developing complications in people with type 1 diabetes? Diabet Med 2020;37:513–521
[1] Messer LH, Berget C, Vigers T, et al. Real world hybrid closed-loop discontinuation: Predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes 2020;21:319–327
[1] Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol 2019; 13:614–626
[1] Avari P, Uduku C, George D, Herrero P, Reddy M, Oliver N. Differences for percentage times in glycemic range between continuous glucose monitoring and capillary blood glucose monitoring in adults with type 1 diabetes: analysis of the REPLACE-BG dataset. Diabetes Technol Ther 2020; 22:222–227
[1] Vigersky RA, McMahon C.The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther 2019;21: 81–85
[1] Livingstone R, Boyle JG, Petrie JR. How tightly controlled do fluctuations in blood glucose levels need to be to reduce the risk of developing complications in people with type 1 diabetes? Diabet Med 2020;37:513–521
[1] Messer LH, Berget C, Vigers T, et al. Real world hybrid closed-loop discontinuation: Predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes 2020;21:319–327
[1] Mayeda L, Katz R, Ahmad I, et al. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res Care 2020;8:e000991
[1] Yoo JH, Choi MS, Ahn J, et al. Association between continuous glucose monitoring-derived time in range, other core metrics, and albuminuria in type 2 diabetes. Diabetes Technol Ther 2020; 22:768–776
[1] Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther 2020;22: 72–78
[1] Adler AI, Stratton IM, Neil HAW, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000;321:412–419
[1] Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139
[1] Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358: 2560–2572
[1] Ismail-Beigi F, Craven T, Banerji MA, et al.; ACCORD trial group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–430
[1] Buse JB, Bain SC, Mann JFE, et al.; LEADER Trial Investigators. Cardiovascular risk reduction with liraglutide: an exploratory mediation analysis of the LEADER trial. Diabetes Care 2020;43: 1546–15
[1] Nathan DM, Cleary PA, Backlund JYC, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653
[1] Nathan DM, Zinman B, Cleary PA, et al.; Diabetes Control and Complications Trial/ Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983-2005). Arch Intern Med 2009;169:1307–1316
[1] Emerging Risk Factors Collaboration; Di Angelantonio E, Kaptoge S, Wormser D, et al. Association of cardiometabolic multimorbidity with mortality. JAMA 2015;314:52–60
[1] Yeung RO, Zhang Y, Luk A, et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a crosssectional study of a prospective cohort. Lancet Diabetes Endocrinol 2014;2:935–943
[1] Sattar N, Rawshani A, Franzen S, et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation 2019;139:2228–2237
[1] Zabala A, Darsalia V, Holzmann MJ, et al. Risk of first stroke in people with type 2 diabetes and its relation to glycaemic control: a nationwide observational study. Diabetes Obes Metab 2020; 22:182–190
[1] Zoungas S, Woodward M, Li Q, et al.; ADVANCE Collaborative group. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia 2014;57: 2465–2474
[1] Skyler JS, Bergenstal R, Bonow RO, et al.; American Diabetes Association; American College of Cardiology Foundation; American Heart Association. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009;32:187–192
[1] Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358: 2545–2559
[1] Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358: 2545–2559
[1] Duckworth WC, Abraira C, Moritz TE, et al.; Investigators of the VADT. The duration of diabetes affects the response to intensive glucose control in type 2 subjects: the VA Diabetes Trial. J Diabetes Complications 2011;25:355–361
[1] Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356–362
[1] Vijan S, Sussman JB, Yudkin JS, Hayward RA. Effect of patients’ risks and preferences on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med 2014;174: 1227–1234
[1] Lee AK, Warren B, Lee CJ, et al. The association of severe hypoglycemia with incident cardiovascular events and mortality in adults with type 2 diabetes. Diabetes Care 2018;41:104–111
- Annexes
Annex 1:
Annex 2: