Streptococcosis
| Site: | EHC | Egyptian Health Council |
| Course: | Aquatic animal medicine Guidelines |
| Book: | Streptococcosis |
| Printed by: | Guest user |
| Date: | Tuesday, 24 December 2024, 1:29 AM |
Description
"last update: 28 April 2024"
- Committee
Committee Chair: Prof. Ahmed M Byomi
The Rapporteur of the Committee: Prof. Dr Mohamed Mohamady Ghanem.
Committee Members: Prof. Nabil yassien; Prof. Ashraf Aldesoky Shamaa; Prof. Amany Abbass; Prof. Dalia Mansour; Dr Essam Sobhy.
Authors: Mohamed Faisal1,2; Amany A. Abbass1 ; Adel A. Shaheen1; Amel M. El Asely1; Eman A. Abd El-Gawad1; Hiam S. Elabd1; Aya F. Matter1; Hadeer A. Youssef1, and Amira M. El-Daim1.
1Department of Aquatic Animal Medicine, Faculty of Veterinary Medicine, Benha University, Egypt.
2College of Veterinary Medicine, Michigan State University, USA.
- Scope
Streptococcosis is a systemic bacterial disease causing significant economic losses for both freshwater, marine and aquarium fishes as well as it has zoonotic importance. Globally, the pathogens greatly impact the cultured Nile tilapia, Oreochromis niloticus causing high mortalities and economic losses.
- Summary
Streptococcosis is the general term for a variety of diseases caused by members of the genus Streptocoocus, lactococcus Enterococcus, Vagococcus. Streptococcus iniae, Streptococcus agalactiae, Streptococcus dysgalactiae and Lactococcus garvieae have great concern that can seriously cause significant losses in the global aquaculture development especially Nile tilapia production. The most common clinical signs of disease are meningoencephalitis, skin lesion, abdominal distension, exophthalmia, and corneal opacity. The disease outbreaks are usually during the summer season and pathogen transmitted mainly by horizontal route. To reduce economic losses and combat disease outbreaks, several preventive and control measures have been developed. Vaccination via oral method is a very promising measure for mass protection of fish against disease compared to other application routes. As well as Strept. iniae chitosan nanoparticle vaccines showed significant improvement in fish immunity and high survivability against the disease.
- Introduction
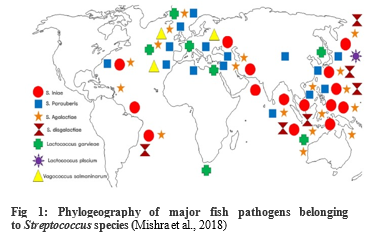
Fish Streptococcosis is one of re-emerging infectious bacterial diseases of freshwater and marine fishes economically affecting the sustainability of aquaculture development worldwide (Fig 1) (Mishra et al., 2018). The bacterium has been isolated from water and from the gastrointestinal (GI) tracts of various vertebrate and invertebrate animals. Therefore, it’s an opportunistic bacterial disease that occur in stressed fishes. (Streptococcosis can occur in warm water, and mainly the causative agent is Strept. iniae, L. garvieae, Strept. agalactiae, and Strept. parauberis, meanwhile cold water streptococcosis (below 15°C) is caused mostly by V. salmoninarum and L. piscium (Domenech et al., 1996).
In Egypt, Strept. iniae was isolated from Nile tilapia (Younes et al., 2019), Strept. agalactiae and Strept. dysagalactiae subsp. dysgalactiae (Abu-Elala et al., 2020), L. garvieae (El-daim et al., 2023a), E. faecalis (Elgohary et al., 2021).
- Etiological agent
◾ Several species have been recorded as a causative agent of streptococcal infection including Streptococcus iniae (first isolation from subcutaneous abscess in Amazon freshwater dolphin in 1970s), Streptococcus agalactiae (Ia, Ib and III), Streptococcus dysagalactiae, Streptococcus ictaluri, Streptococcus uberis, Streptococcus parauberis, and Streptococcus phocae, are considered considerable re-emerging pathogens of farmed and wild fishes (Van Doan et al., 2022).
◾ Genus Streptococcus belongs to the order Lactobacillales (lactic acid bacteria) that are Gram-positive, spherical or ovoid arranged in long or short chain, non-spore-forming, non-motile and facultative anaerobic bacteria.
◾ According to the ability to hemolyze blood, Streptococcus has been classified into three groups: α-hemolytic; β-hemolytic, and γ-hemolytic (Foo et al., 1985).
◾ Streptococcosis is a complex multifactorial disease, depending on species, age, immune status, type of pathogen (species and strain), and environmental conditions. Many unfavorable environmental aspects including high water temperature (more than 28°C), low dissolved oxygen, high pH, ammonia, and high organic content, agricultural wastewater, and external parasitic infestation enhance the severity and incidence of infection.
- Diagnosis
a-Clinical sign and lesions
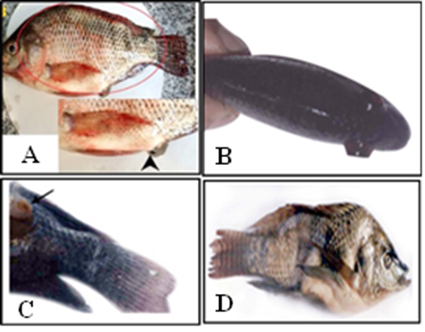
◾The most common clinical signs of streptococcosis are Lethargy, loss of appetite, abnormal behavioral swimming, abdominal distention, severe congestion all over the body, rectal prolapse (Fig 2A), body darkness unilateral or bilateral exophthalmia (Pop eye) (Fig 2B), corneal opacity, abscess like nodule in caudal peduncle and mandible (Fig 2C) and skeletal deformity (Fig 2D) (Abdel-Gawad et al., 2007; El-daim et al., 2023a). Buccal paralysis in the case of S. agalactiae Ib was also observed. Fecal casts or strings are characteristic for streptococcal infection.
◾ Internally, the affected fish may show yellowish to bloody ascitic fluid in the abdominal cavity; pale enlarged liver, dark red spleen; congestion and enlargement of anterior kidneys (El-daim et al., 2023a), fibrinous pericarditis and peritonitis, hemorrhages in the brain, retrobulbar region, intestines, and liver (Sudpraseart et al., 2020).
We should note that these clinical signs are not pathognomonic for streptococcosis because they are not distinct from Lactococcosis caused by L. garvieae & L. piscium, Enterococcosis caused by E. faecalis, Vagococcosis caused by V. salmoninarum.
b-Laboratory diagnosis
Samples: Liver, brain, spleen, and kidneys of affected fish.
I-Presumptive diagnostic assay
Isolation and identification
◾Streptococcous spp are Gram-positive cocci grouped in short to medium-length chains (Fig 3) (Abdel-Gawad et al., 2007), non-motile, catalase negative, oxidase negative, fermentative, with a negative VP response.◾ On 5% sheep blood agar, tryptic soy agar, and brain heart infusion agar incubated at 25 to 35oC for 24 to 48 hrs, small white and umbonate colonies (up to 1 mm) with an entire opaque border are produced.
◾ Strept. dysgalactiae on Colombia blood agar (CBA) demonstrated the presence of pinpoint white colonies (0.5 mm) (Yang and Li, 2009).
◾ RAPID Strep strip, VITEX systems, API 20E STREP, Rapid Strep 32 and ATB Expression System can be carried out, however, incomplete, or incorrect pathogen database may result in improper identification of bacteria.
II-Histopathological examination
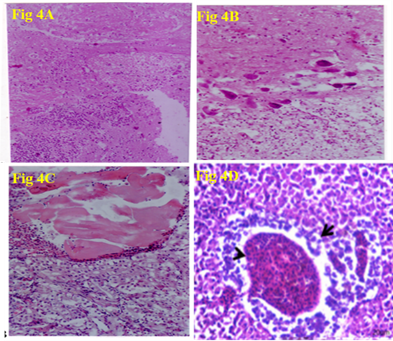
◾ Congestion of the cerebral blood vessels together with focal areas of encephalomalacia (Fig 4A), Galiosis, satillitosis, neural degeneration and neurophagia (Fig 4B), Abscess formation characterized by areas of necrosis surrounded by inflammatory cells was observed in kidneys (Fig 4C) were recorded in Nile tilapia infected with Streptococcous sp (Abdel-Gawad et al., 2007). Liver of Nile tilapia infected with Streptococcus agalactiae revealed dilated hepatic blood vessels (arrowheads), degenerative and necrotic changes of pancreatic epithelium (Fig 4D) (El-daim et al., 2023a).
III-Confirmatory diagnosis
Serological identification
◾Lancefield grouping (A-H and K-V) described serological identification of hemolytic and non-hemolytic bacteria based on group-specific polysaccharides (C antigen). Serological tests, such as the enzyme-linked immunosorbent assay indirect fluorescent antibody are applied for quick detection and identification of bacteria. (Huang et al., 2018).
◾ Strept. iniae and Strept. ictaluri are non-groupable with the Lancefield method (Shoemaker et al. 2017).
Molecular identification
◾ PCR diagnosis of various Streptococcus spp based on the selective amplification of the 16S rRNA, 16S-23S rRNA gene, heat-shock genes (groESL), and tRNA gene intergenic spacer regions (ITSs) is used for confirmative identification (Ortega et al., 2018).
◾ Loop-mediated isothermal amplification method (LAMP) (Wang et al., 2012); Multi Locus Sequence Typing (MLST) (Heckman et al., 2020), and MALDI-TOF-MS (Souter et al., 2021)
- Control of disease
Preventive measures
◾ Application of Standard operating procedures (SOPs) and biosecurity measurements are an effective strategy for limiting disease spread.
◾ Vaccine application in aquaculture is also effective for disease prevention. Formalin killed Strept. agalactiae vaccine via different routes (Linh et al., 2022), DNA vaccines and live attenuated Strept. iniae vaccine (Heckman et al., 2022) were applied. Also, oral delivery of nanoemulsion Streptococcus agalactiae vaccine with bile salt coated by chitosan in Nile tilapia (Suwanbumrung et al., 2023).
◾ In Egypt, there was limited research towards generating a streptococcal commercial vaccine. Montanide adjuvant Strept. agalactiae (Abu-Elala et al., 2019) and polyvalent formalin and autoclaved inactivated vaccine (El-daim et al., 2023b) were used as a trial for protecting Nile tilapia against streptococcosis.
◾ Immunostimulants such as herbal, prebiotics and probiotics have protective effects against Streptococcus sp. infection (Van Doan et al., 2022).
Treatment
◾ Sensitivity test should be done to select the most effective antibiotic to use.
◾ The approved antibiotics that have been used to control streptococcal infection, are oxytetracycline (75 and 100 mg/ kg), 1.5 g erythromycin/ kg diet for fed for 10 to 14 days (Darwish and Hobbs, 2005), and Aquaflor (florfenicol) (15 mg/kg fish per day for 10 consecutive days) (Oliveira et al., 2018).
Zoonotic importance
◾ Strept. agalactiae, and Strept. dysgalactiae, and Strept. iniae were known to be zoonotic for immunocompromised people who handled live fish (Gauthier, 2015).
◾ In humans, handling of infected live and dead fish can result in development of cellulite, endocarditis, meningitis, severe systemic infections, suppurating ulcers, septicemia, arthritis, lymphadenitis (Haenen et al., 2013), and rarely death.
- References
Abdel-Gawad, EA., Abbass, AA., Shaheen, AA. (2007). Streptococcosis in some fishes: characterization of whole cell protein of the prevalent streptococcus spp. Benha Veterinary Medical Journal, 18 (2): 51-64.
Abu-Elala, NM., Reham, M., Abd-Elsalam, Nehal, A., Younis. (2020). Streptococcosis, Lactococcosis and Enterococcosis are potential threats facing cultured Nile tilapia (Oreochomis niloticus) production. Aquaculture Research. 51:4183–4195.
Abu-Elala, NM., Samir, A., Wasfy, M. Elsayed, M. (2019). Efficacy of injectable and immersion vaccines against streptococcal infections in broodstock and offspring of Nile tilapia (Oreochomis niloticus). Fish & Shellfish Immunology, 88: 293–300.
Darwish, A.M., Hobbs, M.S. (2005). Laboratory efficacy of amoxicillin for the control of Streptococcus iniae infection in blue tilapia.” Journal of Aquatic Animal Health 17(2): 197–202.
Domenech, A., Ferna´ndez G. J.F, Pascual, C., Garcia, J.A., Cutuli, M.T., Moreno, M.A., Collins, M.D., Dominguez, L. (1996). Streptococcosis in cultured turbot, Scophthalmus maximus (L.), associated with Streptococcus parauberis. J Fish Dis. 19(1): 33-38.
El-daim A., Abdel Gawad E. A., El Asely A. M., Abbass A.A., Shaheen A. A. (2023b) Immune Responses and Protective Efficacy Against Streptococcosis Following Polyvalent Inactivated Vaccine Injection in the Nile Tilapia, Oreochromis niloticus. Egyptian Journal of Aquatic Biology & Fisheries, 27(3): 625 – 642
El-daim A.M., El Asely A.M., Abdel Gawad E.A., Abbass A.A., Shaheen A.A. (2023a). Prevalence of Streptococcosis-related Mortalities in Farmed Nile Tilapia (Oreochromis niloticus) at Different Life Stages. Egyptian Journal of Veterinary sciences, 54 (5): 949-964
Elgohary, I., Eissa, A.E., Fadel, N.G., Ibrahim, Abd Elatief, J., Mahmoud, M.A. (2021). Bacteriological, molecular, and pathological studies on the Gram‐positive bacteria Aerococcus viridans and Enterococcus faecalis and their effects on Oreochromis niloticus in Egyptian fish farms. Aquaculture Research 52: 2220-2232.
Foo, J.T., Ho, B. and Lam, T.J. (1985). Mass mortality in Siganus canaliculatus due to streptococcal infection. Aquaculture 49: 185–96.
Gauthier, DT. (2015). Bacterial zoonoses of fishes: A review and appraisal of evidence for linkages between fish and human infections. The Veterinary Journal 203: 27-35.
Haenen OLM, Evans JJ, Berthe F. (2013). Bacterial Infections From Aquatic Species: Potential For and Prevention Of Contact Zoonoses. Rev Sci Tech Off Epiz. 32(2):497–507.
Heckman T. I., Shahin K, Henderson E.E., Griffin M. J., Soto E. (2022). Development and efficacy of Streptococcus iniae live-attenuated vaccines in Nile tilapia, Oreochromis niloticus. Fish & Shellfish Immunology,121: 152-162,
Heckman TI, Griffin MJ, Camus AC, LaFrentz BR, Morick D. et al. (2020). Multilocus sequence analysis of diverse Streptococcus iniae isolates indicates an underlying genetic basis for phenotypic heterogeneity. Disease Aquatic Organisms, 17: 141:53-69.
Huang, G., Huang,Y, Zhang, J., Xiang, Y., Yuan, Z., Tam, J., Zheng, J., Liu, T. (2018). Development and evaluation of lateral flow test strips for Fish pathogenic streptococcus agalactiae. Immunology Research and Therapy Journal; 1(1):113.
Linh, N.V.; Dien, L.T.; Dong, H.T.; Khongdee, N.; Hoseinifar, S.H. et al (2022). Efficacy of Different Routes of Formalin-Killed Vaccine Administration on Immunity and Disease Resistance of Nile Tilapia (Oreochromis niloticus) Challenged with Streptococcus agalactiae. Fishes, 7, 398
Locke, J.B.,Aziz, R.K.,Vicknair, M.R., et al. (2008). Streptococcus iniae M-like protein contributes to virulence in fish and is a target for live attenuated vaccine development. PLoS One; 3:e2824.
Mishra, A., Gyu-Hwi, N., Jeong-An, G., Hee-Eun, L., Ara, JO., Heui-Soo, K. (2018). Current Challenges of Streptococcus Infection and Effective Molecular, Cellular, and Environmental Control Methods. Aquaculture molecules and cells, 41(6):495-505.
Oliveira, T.F., Queiroz, G.A., Teixeira, J.P., Figueiredo, H.C. P., Leal, C.A.G. (2018). Recurrent Streptoccoccus agalactiae infection in Nile tilapia (Oreochromis niloticus) treated with florfenicol, Aquaculture 493 , 51-60.
Ortega, C., Garcia, I. R., Fajardo, R., Tapia-Cammas, D., Acosta, J., Avendano-Herrera, R. (2018). First identification and characterization of Streptococcus iniae obtained from tilapia (Oreochromis aureus) farmed in Mexico. Journal of Fish Diseases 41:773–782.
Shoemaker, C. A., D. Xu, and E. Soto. (2017). Streptococcus iniae and Streptococcus agalactiae. Pages 298-313 in P. T. K. Woo and R. Cipriano, editors. Fish viruses and bacteria: pathobiology and protection. CABI, Inc
Sudpraseart, C.,Wang, Pei-Chi., Chen, Shih-Chu. (2020). Phenotype, genotype and pathogenicity of Streptococcus agalactiae isolated from cultured tilapia (Oreochromis spp.) in Taiwan. Journal of fish diseases.747-755.
Suwanbumrung D, Wongkhieo,S., Keaswejjareansuk W, Dechbumroong P., Kamble M.T et al. (2023). Oral delivery of a Streptococcus agalactiae vaccine to Nile tilapia (Oreochromis niloticus) using a novel cationic-based nanoemulsion containing bile salts, Fish & Shellfish Immunology,139: 108913,
Van Doan, H., Soltani, M., Leitão, A., Shafiei, S., Asadi, S., Lymbery, A.J., Ringø, E. (2022). Streptococcosis a Re-Emerging Disease in Aquaculture: Significance and Phytotherapy. Animals, 12, 2443.
Yang, W and Li, A. (2009). Isolation and characterization of Streptococcus dysgalactiae from diseased Acipenser schrenckii, Aquaculture, 294 (1-2):14-17.
Younes, A. M., Alkhateib, Y. G., Alaa El-Din, Z., Abu-Bryka, Mohamed, L, A., El-Sayed M. B. (2019). Genotyping and pathogenicity of Streptococcus iniae strains recovered from cultured Oreochromis niloticus at Kafr El-Shiekh Governorate, Egypt, Egyptian Journal of Aquatic Biology & Fisheries, 23(3): 467 – 474.