Prostate cancer
| Site: | EHC | Egyptian Health Council |
| Course: | Oncology and Hematological Oncology Guidelines |
| Book: | Prostate cancer |
| Printed by: | Guest user |
| Date: | Monday, 23 December 2024, 9:43 PM |
Description
"last update: 28 April 2024"
- Committee
• Chair of the Oncology Committee of
Egyptian health council Guidelines: Prof
Hussein Khaled.
• The Oncology Committee Members: Ebtesam Saad Eldin , Ihab Khalil, Emad Hamada, Fouad Abuotaleb, Hesham Elghazaly, Hesham Tawfik, , Khaled Abdelkarim, Lobna ezz Elarab, Mary Gamal, Mohamed Abdel Mooti, Mohamed Gamil, Nervana Hussein, Ola Khorshid, Omar Sherif Omar, Rasha shaltout, Rasha fahmy , Samir Shehata, Yousri Wasef & Yousri Rostom.
• Chair of the prostate cancer Scientific Committee: Emad Hamada
• The prostate cancer Scientific Group Members: Alaa Meshref , Ehab Khalil , Hatem abu elkassem , Hesham Tawfik , Hussien Khaled , ,Mary Gamal , Nervana hussein .
- Abbreviations
ADT ( Androgen deprivation therapy )
ART (Adjuvant RT)
AS (Active Surveillance)
AUA (American Urological Association)
BRCA1/2 (Breast Cancer Gene)
BT (Brachytherapy)
CBC & differential ( complete blood count & differential)
CT ( computed tomography )
CRPC ( castrate resistant prostate cancer )
DRE ( digital rectal examination )
DT ( doubling time )
dMMR ( deficient mismatch repair )
EAU ( European Association Of Urology )
EBRT ( external beam radiotherapy )
ECOG ( eastern cooperative oncology group )
ESMO ( European Society For Medical Oncology )
HSPC ( hormone sensitive prostate cancer )
HRR ( homologous recombination repair )
HRD (Homologous recombination deficiency)
H-MSI ( high levels of microsatellite instability )
IHD ( ischemic heart disease )
IRF ( intermediate risk factors )
IMRT ( intensity modulated radiation therapy )
IGRT (Image Guided RT)
KFT ( Kidney function test )
LFT ( liver function test )
LHRH ( luteinizing hormone releasing hormone )
LN ( lymph node )
MCRPC ( metastatic castrate resistant prostate cancer )
MDT ( multi disciplinary team )
MHSPC ( metastatic hormone sensitive prostate cancer )
MRI ( magnetic resonance imaging )
MRI-Bx (MRI guided biopsy)
Mp MRI ( multiparametric MRI )
NCCN ( National Comprehensive Cancer Network )
NMCRPC ( non metastatic castrate resistant prostate cancer )
PARP-inhibitors: poly (ADP-ribose) polymerase Inhibitors
Pca (Prostate Cancer)
PET ( positron emission tomography)
PIRADs (Prostate Imaging-Reporting and Data System)
PS ( performance status )
PSA ( prostatic specific antigen )
PSA DT ( PSA doubling time )
PSMA ( prostate specific membrane antigen )
PLND ( pelvic lymph node dissection )
RP ( radical prostatectomy )
RT (radiation therapy)
SBRT ( stereotactic body radiotherapy )
S-RT (Salvage RT)
TRUS ( transrectal ultrasound )
TRUS-Bx (transrectal guided ultrasound biopsy)
TURP ( trans urethral resection of the prostate )
VMAT (Volumetric Modulated Arc Therapy)
- Glossary
Localized prostate cancer
Prostate cancer that has not spread beyond the prostate
Locally Advanced prostate cancer
Patients with T3b or T4 disease on their initial evaluation based upon the presence of presumed extra-prostatic extension and/or seminal vesicle involvement, or invasion of adjacent organs and/or regional LN metastases by radiological investigations
Biochemical recurrence
A rise in serum PSA and not accompanied by signs, symptoms, or radiographic evidence of locally recurrent or disseminated disease. (by conventional Imaging and/or PSMA-PET)
A) PSA persistence/recurrence after RP
Failure of PSA to fall to undetectable levels (PSA persistence) or undetectable PSA after RP with a subsequent detectable PSA that increases on 2 or more determinations (PSA recurrence) or that increases to PSA >0.1 ng/mL.
B) Biochemical recurrence after EBRT with or without hormonal therapy is defined as a PSA rise by 2 ng/mL or more above the nadir PSA
Metastatic Hormone Sensitive Prostate Cancer (MHSPC)
MHSPC is diagnosed when cancer has spread beyond the prostate to the body and serum testosterone levels are typically >50 ng/dL ,Treatment is often effective with low testosterone levels .
MHSPC High volume criteria ( CHAARTED trial )
Presence of visceral metastases
and/or ≥4 bone metastases, including at least one beyond the vertebral bodies and pelvis
MHSPC low volume criteria are defined as those who do not meet the high volume criteria
MHSPC High risk criteria ( LATITUDE trial )
At least two of :
a-Gleason score of ≥ 8
b-Bone metastasis of ≥ 3
c-Presence of Visceral metastasis
MHSPC low risk criteria are defined as those who do not meet the high risk criteria
Non metastatic CRPC
Males who are diagnosed with CRPC at a time when the only manifestation of progressive disease is an increase in serum PSA level, without demonstrable radiographic disease progression (on bone scan and conventional CT) or by PSMA-PET involving specific organs, , with PSA DT ≤10 months, and a serum PSA ≥2 ng/mL.
Metastatic CRPC
Metastatic castration-resistant prostate cancer (CRPC) is advanced prostate cancer with evidence of disease progression and spread to other parts of the body despite castrate levels of serum testosterone (<50 ng/dL) after medical or surgical orchiectomy.
- Executive Summary
This guidance provides a data-supported approach to the diagnosis, risk stratification, treatment and follow up of patients diagnosed with prostate cancer
|
Level Of recommendation |
|
|
1-Screening for prostate cancer |
|
|
Early PSA testing (baseline PSA followed by risk-adapted follow-up) can be offered to men >50 years, men >45 years with a positive family history of prostate cancer, and BRCA1/2 carriers >40 years |
Conditional |
|
2-Work up for newly diagnosed prostate cancer |
|
|
History and physical examination Personal and family history, Physical examination, DRE , Assessment of ECOG performance status should be done |
Strong |
|
Assessment of life expectancy is a very essential tool in the plan of management of prostate cancer , Life expectancy should be estimated using: The WHO’s Life Tables by country |
Strong |
|
Laboratory Studies Base line tumor marker: serum PSA (Total, Free ) is the recommended initial laboratory studies for localized prostate cancer |
Strong |
|
Radiological Studies TRUS is the initial imaging studies for diagnosis of prostate cancer |
|
|
MRI prostate or mpMRI (if available ) is to be used in the staging and characterization of prostate cancer |
Conditional |
|
Radiologists should utilize PI-RADS V 2.1 in the reporting of multi-parametric MRI (mpMRI) imaging |
Strong |
|
Standard MRI techniques should be used for examination of the pelvis and/or abdomen for initial evaluation of intermediate and high / very high risk patients and for planning purposes in radiotherapy protocols |
Strong |
|
Bone imaging is indicated in the initial evaluation of intermediate and high / very high risk patients to exclude skeletal metastasis |
Strong |
|
PSMA-PET if available to be considered as an alternative to standard imaging of bone and soft tissue in high and very high risk patients . |
Conditional |
|
Initial Biopsy Definitive diagnosis of cancer prostate requires 6- 12 core biopsies of the prostate, using a needle under transrectal / transperineal ultrasound guidance. |
Strong |
|
For biopsy-naïve patients who have a suspicious lesion on MRI, clinicians can perform targeted biopsies of the suspicious lesion either cognitive or software guided |
Conditional |
|
3-Risk stratification and Management of Localized / Locally advanced prostate cancer |
|
|
Patients with localized prostate cancer should be classified into very low , low , intermediate ( Favourable and unfavourable) , high and very high risk groups |
Strong |
|
Risk stratification of clinically localized prostate cancer facilitate care decisions and guide clinicians in the implementation of selected management options.. |
Strong |
|
Patients with prostate cancer should be managed through a multidisciplinary team ( Urologist , Medical Oncologist , Radiation oncologist , Radiologist and Pathologist ) |
Strong |
|
If expected patient survival ≥ 10 years,: ▪️ Active surveillance, ▪️ RP , ▪️ EBRT or ▪️ BT mono-therapy. |
Strong |
|
In asymptomatic patients with prostate cancer and < 10 years life expectancy , watchful waiting is recommended |
Strong |
|
According to MDT decision and patient preference; It is recommended to use one of the following options in the management of favourable intermediate risk groups ( Life expectancy ≥ 10 years): ▪️ RP and PLND or ▪️ EBRT alone or ▪️ combined EBRT + BT or ▪️ BT monotherapy or ▪️ Careful active surveillance |
Strong |
|
It is recommended to use one of the following options in the management of favourable intermediate risk prostate cancer (Expected Survival 5-10 Years ): ▪️ EBRT ▪️ BT monotherapy ▪️ Watchful waiting |
Strong |
|
Brachytherapy monotherapy is a recommended option for patients with very low, low, or favorable intermediate-risk prostate cancer and life expectancy > 10 years with acceptable 10-year recurrence-free survival rate for LDR/HDR brachytherapy |
Strong |
|
RP + PLND or EBRT + short course ADT ( 6 months ) are the recommended options for management of unfavourable intermediate risk patients. |
Strong |
|
Long term ADT ( 2- 3 years ) combined with EBRT is the recommended primary treatment for high risk or very high risk prostate cancer patients |
Strong |
|
RP and PLND is a valid option in very selected cases with high or very high risk prostate cancer based on MDT discussion |
Conditional |
|
Locally advanced prostate cancer |
|
|
Neoadjuvant ADT ( 4-6 months ) followed by ADT + EBRT , then ADT for 2 years is the recommended treatment option for patients with locally advanced prostate cancer |
Strong |
|
RP and PLND can be an option in selected cases of locally advanced prostate cancer according to MDT decision |
Conditional |
|
Patients who choose active surveillance program should have regular follow-up with baseline biopsy , serum PSA level , Prostatic MRI and key principles of active surveillance include: PSA every 3months unless there is an earlier clinical indication DRE every 6 months unless there is an earlier clinical indication. Repeat radiological examination +/- Prostatic biopsy if there is a clinical indication |
Conditional |
|
Watchful waiting should involve monitoring with a history and physical exam every 12 months (without surveillance biopsies) until symptoms develop. |
Strong |
|
Radical prostatectomy RP +/- PLND is the recommended therapy for any patient with clinically localized prostate cancer that can be completely excised surgically, Life expectancy of ≥10 years, and has no serious comorbid conditions that would contraindicate an elective operation |
Strong |
|
Extended PLND is recomended when PLND is performed as it provides more complete staging and may cure some patients with microscopic metastases . An extended PLND includes removal of all node-bearing tissue from an area bound by the external iliac vein anteriorly, the pelvic sidewall laterally, the bladder wall medially, the floor of the pelvis posteriorly, Cooper's ligament distally, and the internal iliac artery proximally. |
Strong |
|
Robotic surgery could be done (if available ) in selected university hospitals after gaining sufficient learning curve |
Conditional |
|
Radiotherapy Indications of Post-prostatectomy ART include Adverse pathologic features : Positive margins, Seminal vesicle invasion, Extracapsular extension) or persistent PSA levels (PSA does not fall to undetectable levels). |
Strong |
|
Strong |
|
|
Radiotherapy in prostate cancer is recommended to be in the treatment plan through an expert MDT and should be carried out in a well-equipped centres with trained personnel and adopting advanced EBRT techniques that include: IMRT, VMAT , image-guided (IGRT) and SBRT facilities. |
Good practice statement |
|
Short-term precise hypo-fractionated radiotherapy can be used as it shortens the treatment course significantly while the treatment results are equivalent to those of conventional high-dose radiotherapy. |
Conditional |
|
Conditional |
|
|
Prophylactic nodal radiation should be considered in locally advanced prostate cancer and clinically positive nodes , and it should be dose escalated in the presence of positive nodes by imaging procedures. |
Strong |
|
Androgen deprivation therapy ADT includes LHRH agonist as Goserline or leuprolide , first generation antiandrogen (Bicalutamide) should be given at least 7 days before LHRH agonist only to avoid flare up phenomenon . |
Strong |
|
We recommend against Combined androgen blockade (medical or surgical castration combined with an antiandrogen) as it provides modest to no benefit over castration alone in patients with prostate cancer |
Strong |
|
ADT should not be used as monotherapy in clinically localized prostate cancer unless there is a contraindication to definitive local therapy, such as life expectancy less than 5 years and presence of comorbidities. Under those circumstances, ADT may be an acceptable alternative if the disease is high or very high risk |
Conditional |
|
Follow
Up For patients initially treated with definitive therapy with intent to cure, serum PSA levels should be measured every 3 months for the first 2 years then every 6 months till 5 years and then annually. |
Strong |
|
4- Management of biochemical recurrence |
|
|
Laboratory
Studies Serum PSA (Total, Free ) , PSA doubling time ( PSA DT ) are the recommended laboratory studies for patients with biochemical recurrence |
Strong |
|
Radiological Studies Standard MRI techniques for examination of the pelvis and/or abdomen is recommended as part of workup for recurrence or progression |
Strong |
|
Bone imaging should be considered for the evaluation of the patient post-prostatectomy when there is failure of PSA to fall to undetectable levels, or when there is undetectable PSA after RP with a subsequent detectable PSA that increases on 2 or more subsequent determinations. |
Strong |
|
Bone imaging should be considered for the evaluation of patients with an increasing PSA or positive DRE after RT |
Strong |
|
In patients with a BCR after local therapy, prostate-specific membrane antigen (PSMA)-PET ( if available ) to be done in lieu of conventional imaging or after negative conventional imaging for further evaluation of clinical recurrence. |
Conditional |
|
Treatment of biochemical recurrence Salvage RT in addition to Six months ADT ( concurrent / Adjuvant ) is recommended for patients with BCR following RP and with high-risk features : ( Gleason Grade Group 4 to 5, PSADT ≤ 6months, persistently detectable post-operative PSA, seminal vesicle involvement). |
Strong |
|
Salvage radiation for a detectable prostate-specific antigen (PSA) after RP is more effective when given at lower levels of PSA. |
Strong |
|
Post-prostatectomy SRT is to treat prostate bed ± pelvic LN , where PSA cut-off value for SRT (range: 0.2–0.5 ng/ml) and 0.2 ng/ml is the preferable value |
Conditional |
|
Immediate rather than deferred ADT is recommended in men with biochemical recurrence after Radiotherapy is recommended if there are high-risk features for early metastases, including a clinical Gleason score 8 -10, or an interval to biochemical recurrence ≤18 months after definitive radiotherapy |
Strong |
|
Salvage RP and PLND can be offered in selected cases with biochemical recurrence after Radiotherapy according to MDT decision |
Conditional |
|
|
|
|
History and physical examination |
Good practice statement |
|
Laboratory Studies CBC, KFT’s and LFT’s, Serum Testosterone Level , HbA1c, serum PSA (Total, Free ) , PSA DT , serum cholesterol /LDL & HDL & S triglycerides , thyroid functions are the recommended work up for advanced prostate cancer |
Good Practice statement |
|
Imaging studies Standard CT techniques should be used for examination of the chest , abdomen and pelvis as an initial evaluation of advanced prostate cancer |
Strong |
|
Bone imaging should be considered for the evaluation of patients with advanced prostate cancer |
Strong |
|
PSMA-PET if available to be considered as an alternative to standard imaging of bone and soft tissue in patients with advanced cancer prostate . |
Conditional |
|
Echocardiogram should be done to assess the cardiac condition as it can guide further management |
Strong |
|
Pathological examination Transrectal US Biopsy is recommended in cases with de novo metastatic prostate cancer |
Strong |
|
In previously treated PC with previous biopsy , we recommend against re-biopsy from the prostate in metastatic setting |
Good practice statement |
|
Biopsy from accessible metastatic lesions to identify patients with small cell/neuroendocrine histomorphologic features can be done in patients with metastatic CRPC |
Conditional |
|
Metastatic hormone sensitive prostate cancer |
|
|
Patients with low-volume metastatic HSPC should be considered for ADT and local radiotherapy to the prostate if not previously given |
Strong |
|
ADT plus docetaxel is the standard of care in treatment of patients with high-volume metastatic HSPC |
Strong |
|
Strong |
|
|
Radiation therapy to the prostate should NOT be performed in men with high-volume metastatic disease outside the context of a clinical trial unless for palliative intent |
Strong |
|
|
|
|
Castrate levels of testosterone should be documented in patients with signs of progression, If serum testosterone levels are <50 ng/dL, the patient should undergo disease workup with bone and soft tissue imaging |
Strong |
|
Apalutamide or enzalutamide should be considered for men with non metastatic CRPC |
Strong |
|
Metastatic Castrate Resistant Prostate Cancer |
|
|
Abiraterone acetate plus prednisone + ADT is the standard of care in the management of patients with metastatic CRPC previously treated with Docetaxel |
Strong |
|
Enzalutamide +ADT is the standard of care in the management of patients with metastatic CRPC previously treated with docetaxel and not candidate for Abiraterone acetate + prednisone |
Strong |
|
Docetaxel + ADT is the standard of care in the management of patients with metastatic CRPC not previously treated with Docetaxel |
Strong |
|
Patients being treated for CRPC should be closely monitored with radiologic imaging (CT, bone imaging), PSA tests, and clinical exams for evidence of progression. |
Strong |
|
Urgent MRI of the spine to detect cord compression is very strongly recommended in men with CRPC with vertebral metastases and neurological symptoms |
Strong |
|
6-Special considerations |
|
|
Docetaxel should be avoided in patients with ECOG PS≥ 2, IHD, presence of comorbidities, grade III/IV peripheral neuropathy , Absolute neutrophil count < 1000/mm3 |
Strong |
|
Apalutamide should be avoided in patients with recent cardiovascular disease or hypothyroidism . |
Strong |
|
Enzalutamide should be avoided in seizure prone patients or with history of seizures |
Strong |
|
Abiraterone should be avoided in patients with uncontrolled diabetes , hepatic impairment , cardiovascular disease |
Strong |
|
Therapy should be continued until clinical progression or intolerable toxicity |
Strong |
|
Palliative RT is recommended for symptomatic control and prevention of complications from metastatic lesions as bone or brain . |
Strong |
|
Bisphosphonate or denosumab is recommended in patients with bone metastases from CRPC at risk for clinically significant skeletal-related events (SREs) |
Strong |
|
The use of a second AR inhibitor (abiraterone after enzalutamide or vice versa) is not recommended |
Strong |
|
Germline testing for BRCA2 and genes associated with cancer predisposition syndromes can be done in patients with positive family history of cancer . |
Conditional |
|
Tumor testing for homologous recombination genes and mismatch repair defects (or microsatellite instability) can be considered in patients with mCRPC |
Conditional |
|
Small cell/neuroendocrine carcinoma of the prostate should be considered in patients with disease that no longer responds to ADT and are positive for metastases. These relatively rare tumors are associated with low PSA levels despite large metastatic burden and visceral disease. |
Strong |
|
Etoposite / platinum is the standard of care in the management of small cell neuroendocrine tumors of the prostate |
Strong |
|
Life style measures is recommended to maintain bone health are recommended for men on ADT: weight-bearing exercise, stop smoking , adequate calcium intake and vitamin D status |
Strong |
- Introduction
Prostate cancer is the fourth most common cancer in Egypt , with estimated number of new cases per year about 5181 ( 7%) ( 1 )
Organ confined disease , locoregional metastasis , Distant metastasis are presented in 80 % , 15% , 5% of cases with a 5 year overall survival 90-99% , 60-80%, 30-40% respectively ( 2 )
➡️Scope of the Guidelines
These guidelines are developed to improve the quality of care for prostate cancer patients Via providing a uniform standard of care across the country to help in early diagnosis , risk stratification and treatment for prostate cancer , with less aggressive treatment options and improved clinical outcomes. These guidelines cover primary diagnosis, staging, treatment and follow-up of prostate cancer patients.
➡️Target audience
Clinicians who are involved in the care and treatment of patients with prostate cancer, including medical oncologists, radiation oncologists, clinical oncologist, urologists , surgeons, interventional radiologists, radiologists, pathologists, and palliative care specialists.
- Methodology
▪️ A comprehensive search for guidelines was undertaken to identify the most
relevant guidelines to consider for adaptation.
▪️ inclusion/exclusion criteria followed in the search and retrieval of
guidelines to be adapted:
- Selecting only evidence-based guidelines (guideline must include a
report on systematic literature searches and explicit links between
individual recommendations and their supporting evidence).
- Selecting only national and/or international guidelines.
- Specific range of dates for publication (using Guidelines published or
updated 2015 and later).
- Selecting peer reviewed publications only.
- Selecting guidelines written in English language.
- Excluding guidelines written by a single author not on behalf of an
organization in order to be valid and comprehensive, a guideline
ideally requires multidisciplinary input.
- Excluding guidelines published without references as the panel needs
to know whether a thorough literature review was conducted and
whether current evidence was used in the preparation of the
recommendations.
▪️ All retrieved Guidelines were screened and appraised using AGREE II
instrument (www.agreetrust.org) by at least two members. the panel decided
a cut-off point or rank the guidelines (any guideline scoring above 50% on
the rigour dimension was retained)
The NCCN , ESMO , AUA , EAU guidelines are the main sources used while formulating the national guidelines for prostate cancer .
➡️Evidence assessment
According to WHO handbook for Guidelines we used the GRADE (Grading
of Recommendations, Assessment, Development and Evaluation) approach
to assess the quality of a body of evidence, develop and report
recommendations. GRADE methods are used by WHO because these
represent internationally agreed standards for making transparent
recommendations. Detailed information on GRADE is available through the
on the following sites:
▪️ GRADE working group: http://www.gradeworkingroup.org
▪️ GRADE online training modules: http://cebgrade.mcmaster.ca/
▪️ GRADE profile software: http://ims.cochrane.org/revman/gradepro
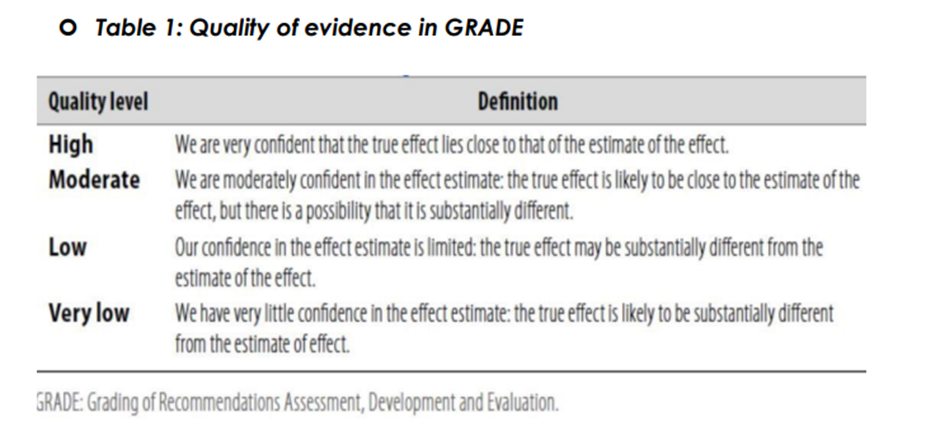
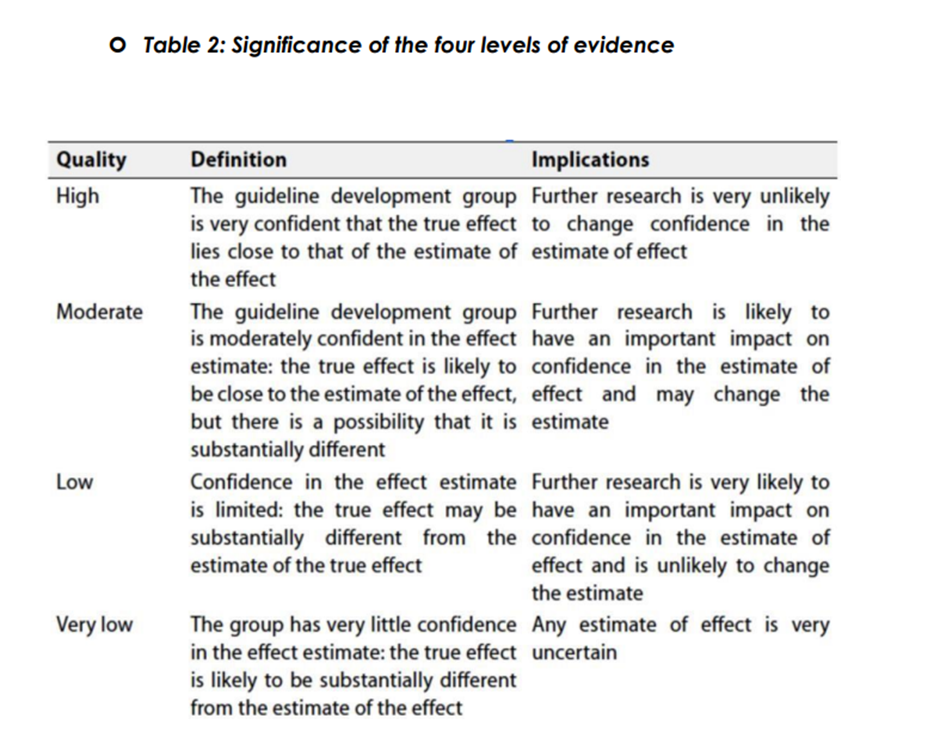
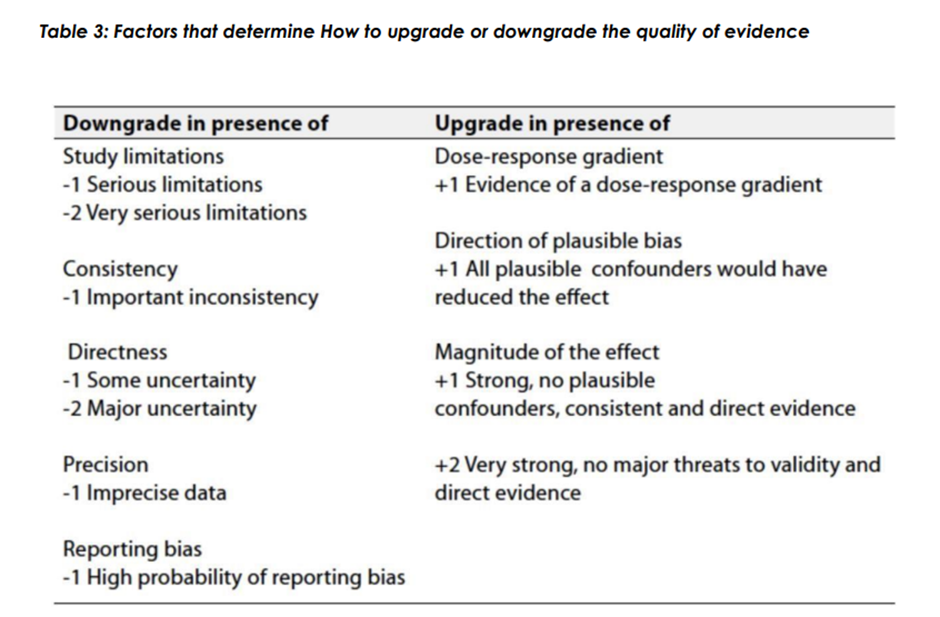
▪️ Table 1: Quality of evidence in GRADE
➡️The strength of the recommendation
The strength of a recommendation communicates the importance of adherence to the recommendation:
▪️ Strong recommendations
With strong recommendations, the guideline communicates the message that
the desirable effects of adherence to the recommendation outweigh the
undesirable effects. This means that in most situations the recommendation
can be adopted as policy.
▪️ Conditional recommendations
These are made when there is greater uncertainty about the four factors
above or if local adaptation must account for a greater variety in values and
preferences, or when resource use makes the intervention suitable for some,
but not for other locations. This means that there is a need for substantial
debate and involvement of stakeholders before this recommendation can be
adopted as policy.
➡️When not to make recommendations.
When there is lack of evidence on the effectiveness of an intervention, it may
be appropriate not to make a recommendation- Recommendations
1- Screening for prostate cancer
Early PSA testing (baseline PSA followed by risk-adapted follow-up) can be offered to men >50 years, men >45 years with a positive family history of prostate cancer, and BRCA1/2 carriers >40 years
Conditional recommendation , moderate quality level of evidence (Randomized Study ) 3
2- Work up for newly diagnosed prostate cancer
➡️History and physical examination
Personal and family history, Physical examination, DRE , Assessment of ECOG performance status should be done
Strong recommendation, high quality level of evidence (prostate cancer prevention trial ) 4
Assessment of life expectancy is a very essential tool in the plan of management of prostate cancer , Life expectancy should be estimated using: The WHO’s Life Tables by country
Strong recommendation, moderate quality level of evidence ( Global Health Observatory data repository) 5
Base line tumor marker: serum PSA (Total, Free ) is the recommended initial laboratory studies for localized prostate cancer
Strong recommendation, high quality level of evidence ( Systematic Review , comparative study ) 6,7
➡️Radiological Studies
TRUS is the initial imaging studies for diagnosis of prostate cancer,
Strong recommendation, high quality level of evidence (Systematic Review ) 6
MRI prostate or mpMRI ( if available ) is to be used in the staging and characterization of prostate cancer
Conditional recommendation, high quality evidence ( prospective study , Meta analysis ) 8,9
Radiologists should utilize PI-RADS V 2.1 in the reporting of multi-parametric MRI (mpMRI) imaging
Strong Recommendation, High quality Evidence Level ( Systematic Review ) 6
Strong Recommendation , high quality Evidence Level (Diagnostic meta analysis) 9
Bone imaging is indicated in the initial evaluation of intermediate and high / very high risk patients to exclude skeletal metastasis
Strong recommendation, high quality evidence(retrospective analysis ) 10
Conditional recommendation, high quality evidence(retrospective analysis )11
➡️Initial Biopsy
Definitive diagnosis of cancer prostate requires 6-12 core biopsies of the prostate, using a needle under transrectal / transperineal ultrasound TRUS guidance.
Strong Recommendation; high quality Evidence Level (confirmatory study , prospective Comparative analysis ) 12, 13
For biopsy-naïve patients who have a suspicious lesion on MRI, clinicians can perform targeted biopsies of the suspicious lesion either cognitive or software guided
Conditional Recommendation , high quality Evidence Level ( prospective multicenter study , Comparative study ) 14 , 15
3- Risk stratification and Management of Localized / Locally advanced prostate cancer
Patients with localized prostate cancer should be classified into very low , low , intermediate ( Favourable and unfavourable) , high and very high risk groups
Strong Recommendation , high quality Evidence Level ( Retrospective analysis ) 16
Risk stratification of clinically localized prostate cancer facilitate care decisions and guide clinicians in the implementation of selected management options..
Strong Recommendation , high quality Evidence Level (Systematic Review) 17
Patients with prostate cancer should be managed through a multidisciplinary team ( Urologist , medical Oncologist , Radiation oncologist , Radiologist , and Pathologist )
Strong Recommendation , high quality Evidence Level ( Retrospective review ) , 18
It is Recommended to use one of the following options in the management of very low/low risk groups (according to MDT decision and patient preference):
If expected patient survival ≥ 10 years,:
▪️ Active surveillance or
▪️ RP or
▪️ EBRT or
▪️ BT mono-therapy
Strong Recommendation , high quality Evidence Level ( Population based validation ) 19
In asymptomatic patients with prostate cancer and limited life expectancy , watchful waiting is recommended
Strong Recommendation, high quality Evidence Level ( Systematic review ) ,17
According to MDT decision and patient preference; It is recommended to use one of the following options in the management of favourable intermediate risk groups ( Life expectancy ≥ 10 years):
▪️ RP and PLND or
▪️ EBRT alone or
▪️ combined EBRT + BT or
▪️ BT monotherapy or
▪️ Careful active surveillance
Strong Recommendation, high quality Evidence Level , ( Systematic review , Retrospective analysis ) 17, 20
It is recommended to use one of the following options in the management of favourable intermediate risk prostate cancer (Expected Survival 5-10 Years ):
▪️ EBRT
▪️ BT monotherapy
▪️ Watchful waiting
Strong Recommendation , high quality Evidence Level (retrospective analysis) 20
Brachytherapy monotherapy is a recommended option for patients with very low, low, or favorable intermediate-risk prostate cancer and life expectancy > 10 years with acceptable 10-year recurrence-free survival rate for LDR/HDR brachytherapy
Strong Recommendation , high quality Evidence , ( Literature review ), 21
RP + PLND or EBRT + short course ADT ( 6 months ) are the recommended options for management of unfavourable intermediate risk patients.
Strong Recommendation , high quality Evidence Level ): ( Systematic review , retrospective analysis )17 , 20
Long term ADT ( 2- 3 years ) combined with EBRT is the recommended primary treatment for high risk or very high risk prostate cancer patients
Strong Recommendation , high quality Evidence Level ( Randomized trial ) 22
RP and PLND is a valid option in very selected cases with high or very high risk prostate cancer based on MDT discussion
Conditional recommendation , high quality level ( Retrospective analysis ) 23
➡️Locally advanced prostate cancer
Neoadjuvant ADT ( 4-6 months ) followed by ADT + EBRT , then ADT for 2 years is the recommended treatment option for patients with locally advanced prostate cancer
Strong recommendation , high quality level ( Randomized trial ) 24
RP and PLND can be an option in selected cases of locally advanced prostate cancer according to MDT decision
Conditional recommendation , high quality level ( Retrospective analysis ) 23
Patients who choose active surveillance program should have regular follow-up with baseline biopsy , serum PSA level , Prostatic MRI and key principles of active surveillance include:
PSA every 3months unless there is an earlier clinical indication
DRE every 6 months unless there is an earlier clinical indication.
Radiological examination +/- Prostatic biopsy if there is a clinical indication
Conditional recommendation, moderate quality evidence ( systematic review ), 25
Watchful waiting involves monitoring with a history and physical exam every 12 months (without surveillance biopsies) until symptoms develop.
Strong recommendation, high quality evidence ( prospective study , cancer epidemiology study ) 26, 27
➡️Radical prostatectomy
RP +/- PLND is the recommended therapy for any patient with clinically localized prostate cancer that can be completely excised surgically, Life expectancy of ≥10 years, and has no serious comorbid conditions that would contraindicate an elective operation
Strong recommendation, high quality evidence (retrospective analysis ), 28
Extended PLND is recommended when PLND is performed as it provides more complete staging and may cure some patients with microscopic metastases . An extended PLND includes removal of all node-bearing tissue from an area bound by the external iliac vein anteriorly, the pelvic sidewall laterally, the bladder wall medially, the floor of the pelvis posteriorly, Cooper's ligament distally, and the internal iliac artery proximally.
Strong recommendation, high quality evidence (systematic review ), 29
Robotic surgery could be done (if available ) in selected university hospitals after gaining sufficient learning curve
Conditional recommendation , high quality evidence ( retrospective analysis )30
➡️Radiotherapy
Indications of Post-prostatectomy ART include Adverse pathologic features : Positive margins, Seminal vesicle invasion and Extracapsular extension or persistent PSA levels (PSA does not fall to undetectable levels).
Strong recommendation, high quality evidence ( randomized clinical trial ), 31
Radiotherapy is one of the recommended modalities of radical therapy for localized prostate cancer patients without severe complications where the results of definitive radiotherapy are comparable to radical prostatectomy for patients with similar recurrence risk. Prospective analysis
Strong recommendation, high quality evidence ( Prospective analysis ), 32
Radiotherapy in prostate cancer is recommended to be in the treatment plan through expert MDT and should be carried out in a well-equipped centres with trained personnel and adopting advanced EBRT techniques that include: IMRT, VMAT , image-guided (IGRT) and SBRT facilities.
➡️Good statement practice
Short-term precise hypo-fractionated radiotherapy can be used as it shortens the treatment course significantly while the treatment results are equivalent to those of conventional high-dose radiotherapy.
Conditional recommendation, high quality evidence ( Systematic review, single institution experience ), 33, 34
Addition of a focal boost to the intra-prostatic lesion can be used as it improved disease free survival for patients with localized intermediate- and high-risk prostate cancer without impacting toxicity and quality of life.
Conditional recommendation , high quality evidence ( randomized trial ), 35
Prophylactic nodal radiation should be considered in locally advanced prostate cancer and clinically positive nodes , it should be dose escalated in the presence of positive nodes by imaging procedures.
Strong recommendation, high quality evidence, ( Randomized trial ) , 36
➡️Androgen deprivation therapy
ADT includes LHRH agonist as Goserline or leuprolide , first generation antiandrogen (Bicalutamide) should be given at least 7 days before LHRH agonist only to avoid flare up phenomenon .
Strong recommendation, high quality evidence (population based cohort study ) , 37
We recommend against Combined androgen blockade (medical or surgical castration combined with an antiandrogen) as it provides modest to no benefit over castration alone in patients with prostate cancer
Strong recommendation, high quality evidence ( randomized controlled trials ) ,38
ADT should not be used as monotherapy in clinically localized prostate cancer unless there is a contraindication to definitive local therapy, such as life expectancy less than 5 years and presence of comorbidities. Under those circumstances, ADT may be an acceptable alternative if the disease is high or very high risk
Conditional recommendation, high quality evidence (overview of randomized trials), 39
Follow Up
Strong recommendation, moderate quality level of evidence (prostate cancer prevention trial ) 4
4-Management of biochemical recurrence
Serum PSA (Total, Free ) and PSA doubling time ( PSA DT ) are the laboratory studies for patients with biochemical recurrence
Strong Recommendation , high quality Evidence ( Comparative study ), 7
➡️Radiological Studies
Standard MRI techniques for examination of the pelvis and/or abdomen is recommended as part of workup for recurrence or progression
Strong Recommendation , high quality Evidence Level (Diagnostic meta analysis) 9
Bone imaging should be considered for the evaluation of the patient post-prostatectomy when there is failure of PSA to fall to undetectable levels, or when there is undetectable PSA after RP with a subsequent detectable PSA that increases on 2 or more subsequent determinations.
Strong recommendation, high quality evidence(retrospective analysis ) 10
Strong recommendation, high quality evidence(retrospective analysis ) 10
In patients with a BCR after local therapy, prostate-specific membrane antigen (PSMA)-PET ( if available ) to be done in lieu of conventional imaging or after negative conventional imaging for further evaluation of clinical recurrence.
Conditional Recommendation, high quality level ( Systematic Review ) , 17
➡️ Treatment of Biochemical Recurrence
Salvage RT in addition to Six months ADT ( concurrent / Adjuvant ) is recommended for patients with BCR following RP and with high-risk features :
( Gleason Grade Group 4 to 5, PSADT ≤ 6months, persistently detectable post-operative PSA, seminal vesicle involvement).
Strong Recommendation, high quality level ( randomized trial ) 40
Salvage radiation for a detectable prostate-specific antigen (PSA) after RP is more effective when given at lower levels of PSA.
Strong Recommendation, high quality level ( Systematic Review ) , 17
Post-prostatectomy SRT is to treat prostate bed ± pelvic LN , where PSA cut-off value for SRT (range: 0.2–0.5 ng/ml) and 0.2 ng/ml is the preferable value
Conditional recommendation, high quality evidence ( retrospective analysis ),41
Immediate rather than deferred ADT is recommended in men with biochemical recurrence after Radiotherapy is recommended if there are high-risk features for early metastases, including a clinical Gleason score 8 -10, or an interval to biochemical recurrence ≤18 months after definitive radiotherapy
Strong recommendation , high quality level ( Randomized trial ) 42
Salvage RP and PLND can be offered in selected cases with biochemical recurrence after Radiotherapy according to MDT decision
Conditional recommendation , high quality level ( Retrospective analysis ) 23
5- Management of
A)Metastatic Hormone Sensitive ,
B)Non Metastatic Castrate Resistant ,
C)Metastatic Castrate Resistant Prostate Cancer
➡️History and physical examination
Including assessment of ECOG Performance status , Presence of peripheral neuropathy , History of seizures or cerebrovascular problems , History of cardiovascular disease and other comorbidities and Risk of fall & fractures
Good practice statement
➡️Laboratory Studies
CBC, KFT’s and LFT’s, Serum Testosterone Level , HbA1c, serum PSA (Total, Free ) , PSA doubling time ( PSA DT ) , serum cholesterol /LDL & HDL & S triglycerides are the recommended work up for metastatic prostate cancer
Good practice statement
➡️Imaging studies
Standard CT techniques should be used for examination of the chest , abdomen and pelvis as an initial evaluation of advanced prostate cancer
Strong Recommendation , high quality Evidence ( Diagnostic meta analysis) 9
Bone imaging should be considered for the evaluation of patients with advanced prostate cancer
Strong recommendation, high quality evidence(retrospective analysis ) 10
PSMA-PET if available to be considered as an alternative to standard imaging of bone and soft tissue in patients with advanced cancer prostate .
Conditional recommendation, high quality evidence(retrospective analysis )11
Echocardiogram should be done to assess the cardiac condition as it can guide further management
Good practice statement
➡️Pathological examination
Transrectal US Biopsy is recommended in cases with de novo metastatic prostate cancer
Strong recommendation, high quality level of evidence (Systematic Review ) 6
In previously treated PC with previous biopsy , we recommend against re-biopsy from the prostate in metastatic setting
Good practice statement
Biopsy from accessible metastatic lesions to identify patients with small cell/neuroendocrine histomorphologic features can be done in patients with metastatic CRPC
Conditional recommendation , strong quality level ( prospective analysis ) 43
A)Metastatic hormone sensitive prostate cancer
Patients with low-volume metastatic HSPC should be considered for ADT and local radiotherapy to the prostate if not previously given
Strong recommendation , high quality level ( Randomized clinical trial ) 44
ADT plus docetaxel is the standard of care in treatment of patients with high-volume metastatic HSPC
Strong recommendation , high quality Evidence (randomized clinical trial) ,45
ADT plus Apalutamide or Enzalutamide is the standard of care in treatment of patients with high-volume metastatic HSPC who are not candidate for docetaxel
Strong recommendation , high quality Evidence (Randomized clinical trials ) 46,47,48
Radiation therapy to the prostate should NOT be performed in men with high-volume metastatic disease outside the context of a clinical trial unless for palliative intent
Good practice statement
B)Non Metastatic Castrate Resistant Prostate Cancer
Castrate levels of testosterone should be documented in patients with signs of progression, If serum testosterone levels are <50 ng/dL, the patient should undergo disease workup with bone and soft tissue imaging
Strong recommendation , high quality level ( Literature review ), 49
Apalutamide or enzalutamide should be considered for men with non metastatic CRPC
Strong recommendation , high quality level ( Randomized clinical trials ) 50, 51
C)Metastatic Castrate Resistant Prostate Cancer
Abiraterone acetate plus prednisone + ADT is the standard of care in the management of patients with metastatic CRPC previously treated with Docetaxel
Strong recommendation , high quality level ( Randomized clinical trial ) 52
Enzalutamide +ADT is the standard of care in the management of patients with metastatic CRPC previously treated with docetaxel and not candidate for Abiraterone acetate + prednisone
Strong recommendation , high quality level ( Randomized clinical trials ) 53,54
Docetaxel + ADT is the standard of care in the management of patients with metastatic CRPC not previously treated with Docetaxel
Strong recommendation , high quality level (literature review ), 55
Patients being treated for CRPC should be closely monitored with radiologic imaging (CT, bone imaging), PSA tests, and clinical exams for evidence of progression.
Strong recommendation, high quality evidence(retrospective analysis ) 10
Urgent MRI of the spine to detect cord compression is very strongly recommended in men with CRPC with vertebral metastases and neurological symptoms
Strong recommendation , high quality Evidence (Systematic review ) ,56
6 - Special considerations
Docetaxel should be avoided in patients with ECOG PS≥ 2, IHD, presence of comorbidities, grade III/IV peripheral neuropathy , Absolute neutrophil count < 1000/mm3
Strong recommendation , high quality level ( randomized clinical trial ) 45
Apalutamide should be avoided in patients with recent cardiovascular disease or hypothyroidism .
Strong recommendation , high quality Evidence ( randomized clinical trial ) 46
Enzalutamide should be avoided in seizure prone patients or with history of seizures
Strong recommendation , high quality Evidence ( randomized clinical trial ) 47, 48
Abiraterone should be avoided in patients with uncontrolled diabetes , hepatic impairment , cardiovascular disease
Strong recommendation , high quality Evidence ( randomized clinical trial ) 52
Therapy should be continued until clinical progression or intolerable toxicity
Strong recommendation , high quality Evidence (randomized clinical trials )45, 47, 48, 52
Palliative RT is recommended for symptomatic control and prevention of complications from metastatic lesions as bone or brain .
Strong recommendation , high quality Evidence (Systematic review ), 57
Bisphosphonate or denosumab is recommended In patients with bone metastases from CRPC at risk for clinically significant skeletal-related events (SREs)
Strong recommendation , high quality Evidence ( Randomized trial ),58
The use of a second AR inhibitor (abiraterone after enzalutamide or vice versa) is not recommended
Strong recommendation , high quality level ( Randomized trial ) 59
Germline testing for BRCA2 and genes associated with cancer predisposition syndromes can be done in patients with positive family history of cancer .
Conditional recommendation , high quality Evidence (comparative study ) 60
Tumor testing for homologous recombination genes and mismatch repair defects (or microsatellite instability) can be considered in patients with mCRPC
Conditional recommendation , high quality Evidence (Randomized trial ) 61
Small cell/neuroendocrine carcinoma of the prostate should be considered in patients with disease that no longer responds to ADT and are positive for metastases. These relatively rare tumors are associated with low PSA levels despite large metastatic burden and visceral disease.
Strong recommendation , high quality Evidence ( Retrospective analysis ), 62
Etoposite / platinum is the standard of care in the management of small cell neuroendocrine tumors of the prostate
Strong recommendation , high quality Evidence ( retrospective analysis ), 63
Life style measures is recommended to maintain bone health are recommended for men on ADT: weight-bearing exercise, stop smoking , adequate calcium intake and vitamin D status
Strong recommendation , high quality Evidence ( Retrospective analysis ), 64
Clinical indicators for monitoring
For patients newly diagnosed with prostate cancer , Transrectal U/S guided biopsy from prostate , Total/ free PSA , imaging studies should be done
For patients initially treated with definitive therapy with intent to cure, serum PSA levels should be measured.
For patients who are on treatment , Regular PSA levels and radiological assessment upon indication should be done
➡️Research Gaps
Head to Head Comparative study between different novel hormonal treatment in the metastatic setting with overall survival , r PFS and PFS 2 as endpoints together with the safety profile for each
Head to Head comparative study between Triplet and Doublet therapy in metastatic HSPC and nm CRPC in terms of OS , PFS , safety profile
➡️Update of this guideline
This guideline will be updated whenever there is new evidence.
- References
1- The global cancer observatory , Globocan 2022
2- Rebello et al , Nature review 2022
3- Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027e2035.
4- Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk:results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98:529e534
5- Life Tables By Country. World Health Organization; Available at: http://apps.who.int/gho/data/view.main. Accessed November 14, 2021.
6- Wei JT, Barocas D, Carlsson S, et al. Early detection of prostate cancer: AUA/SUO guideline part I: prostate cancer screening. J Urol. 2023;210(1):45-53.
7- Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate specific antigen in serum as a screening test for prostate cancer. N Engl J Med 1991;324:1156-1161
8- Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol 2011;186:1818-1824.
9- de Rooij M, Hamoen EH, Witjes JA, et al. Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-analysis. Eur Urol 2016;70:233-245.
10- Merdan S, Womble PR, Miller DC, et al. Toward better use of bone scans among men with early-stage prostate cancer. Urology 2014;84:793- 798.
11- Afshar-Oromieh A, Avtzi E, Giesel FL, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2015;42:197-209.
12- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815e822
13- Hung, W. P. L., et al. "Transrectal vs. transperineal prostate biopsy under local anaesthesia: Prospective comparative analysis of cancer detection, safety and tolerability using patient-reported outcome measures at a single centre." The 35th Annual European Association of Urology Congress (EAU20). Elservier
14- Rouviere O, Puech P, Renard-Penna R, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019;20:100e109
15- Siddiqui, M. M., Rais-Bahrami, S., Turkbey, B., George, A. K., Rothwax, J., Shakir, N., ... & Pinto, P. A. (2015). Comparison of MR/ultrasound fusion–guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. Jama, 313(4), 390-397.
16- Reese AC, Pierorazio PM, Han M, Partin AW. Contemporary evaluation of the National Comprehensive Cancer Network prostate cancer risk classification system. Urology 2012;80:1075-1079.
17- Eastham JA, Auffenberg GB, Barocas DA, et al. Clinically localized prostate cancer: AUA/ASTRO guideline, part I: introduction, risk assessment, staging, and risk-based management. J Urol. 2022;208(1):10-18
18- Hussein, A. A., Iqbal, U., Jing, Z., Ramahi, Y., Houenstein, H., Newman, & Guru, K. A. (2023). Impact of an NCCN-Compliant Multidisciplinary Conference on Treatment Decisions for Localized Prostate Cancer. Journal of the National Comprehensive Cancer Network, 21(4), 359-365.
19- Pompe RS, Davis-Bondarenko H, Zaffuto E, et al. Population-based validation of the 2014 ISUP Gleason grade groups in patients treated with radical prostatectomy, brachytherapy, external beam radiation, or no local treatment. Prostate 2017;77:686-693
20- Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol 2013;64:895-902
21- Pascoe C, Duncan C, Lamb BW, et al. Current management of radiation cystitis: a review and practical guide to clinical management. BJU Int. 2019;123(4):585-594.
22- Nabid A, Carrier N, Martin AG, et al. Duration of androgen deprivation therapy in high-risk prostate cancer: a randomized phase III trial. Eur Urol. 2018;74:432e441.
23- Pierorazio PM, Ross AE, Lin BM, et al. Preoperative characteristics of high-Gleason disease predictive of favourable pathological and clinical outcomes at radical prostatectomy. BJU Int 2012;110:1122-1128
24- Hanks GE, Pajak TF, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003;21:3972e3978.
25- Ganz PA, Barry JM, Burke W, et al. NIH State-of-the-Science Conference Statement: Role of active surveillance in the management of men with localized prostate cancer. NIH Consens State Sci Statements 2011;28:1-27.
26- Johansson JE, Holmberg L, Johansson S, et al. Fifteen-year survival in prostate cancer. A prospective, population-based study in Sweden. JAMA 1997;277:467-471.
27- Use of active surveillance or watchful waiting for low risk prostate cancer and management trends across risk groups in the united states , 2010-2015, Brandon A Mahal et al .JAMA 2019.
28- Stephenson AJ, Kattan MW, Eastham JA, et al. Prostate cancer specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol 2009;27:4300-4305.
29- Fossati N, Willemse PPM, Van den Broeck T, et al. The benefits and harms of different extents of lymph node dissection during radical prostatectomy for prostate cancer: A systematic review. Eur Urol 2017;72:84-109.
30- Francesco Esperto, Loris Cacciatore, Atonio Testa , et al . Impact of robotic technologies on prostate cancer patients ‘ choice for radical treatment . J pers Med 2023
31- Thompson IM , Tangen CM , Paradelo J , et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of prostate cancer metastasis and improved survival : Long term follow up of a randomized clinical trial .J Urol 2009;181:956-962
32- Juanita M. Crook M.D., F.R.C.P.C., et al . Radiotherapy for localized prostate carcinoma. The correlation of pretreatment prostate specific antigen and nadir prostate specific antigen with outcome as assessed by systematic biopsy and serum prostate specific antigen ; Cancer :328-336
33- Hegazy MW, Mahmood RI, AI Otaibi MF, Khalil EM .Hypofractionated volumetric Modulated Arc Radiotherapy with simultaneous Elective Nodal irradiation is feasible in prostate cancer patients : A single institutional experience .J Egypt Natl Canc Inst.2016 , jun;28(2):101-10
34- Jackson WC, Silva J, Hartman HE, et al. Stereotactic body radiation therapy for localized prostate cancer: a systematic review and meta analysis of over 6,000 patients treated on prospective studies. Int J Radiat Oncol Biol Phys. 2019; 104(4):778-789.
35- Kerkmeijer LGW, Groen VH, Pos FJ, et al .Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J Clin Oncol. 2021 Mar 1;39(7):787-796.
36- Murthy V, et al. J Clin Oncol 2021;39:1234-1242.
37- Lu-Yao GL, Albertsen PC, Moore DF, et al. Fifteen-year survival outcomes following primary androgen-deprivation therapy for localized prostate cancer. JAMA Intern Med 2014;174:1460-1467.
38- McLeod DG, Iversen P, See WA, et al. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int 2006;97:247-254.
39- Maximum androgen blockade in advanced prostate cancer: an overview of 22 randomised trials with 3283 deaths in 5710 patients. Prostate Cancer Trialists' Collaborative Group. Lancet. 1995 Jul 29;346(8970):265-9.
40- Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol 2016;17:747-756.
41- Lisanne F et al. Salvage radiotherapy after radical prostatectomy : Long term results of urinary incontinence , toxicity and treatment outcomes.Clin Trans Radiat Oncol 2018 ;11 :26-32
42- Chesne GM, Woo HH, Bassett JK, et al. Timing of androgen deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol 2016;17:727-737.
43- Liu Z et al . Application of metastatic biopsy based on “ When , who, where , how (4W1H)” principle in diagnosis and treatment of metastatic castration resistant prostate cancer .Transl Androl Urol 2021;10(4):1723-1733
44- Parker CC, James ND, Brawley CD, et al , Systemic Therapy for Advanced or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018 Dec 1;392(10162):2353-2366.
45- Kyriakopoulos CE, Chen YH, Carducci MA, et al , . Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol. 2018 Apr 10;36(11):1080-1087.
46- Chi KN, Agarwal N, Anders Bjartell, et al , Apalutamide for metastatic castration sensitive prostate cancer , Titan trial . The New England Journal of Medicine. 2019;381(1):13-24.
47- Armstrong AJ et al. A randomized phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone sensitive prostate cancer , Arches trial . J Clin Oncol 2022; 40(15): 1616–1622
49- Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630e642.
50- Apalutamide and overall survival in non metastatic castrate resistant prostate cancer , Spartan trial , Ann Oncol. 2019 Nov; 30(11): 1813-1820.
51- Sternberg CN, Fizazi K, Saad F, et al , PROSPER Investigators. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med. 2020 Jun 4;382(23):2197-2206.
52- Karim Fizazi, Scher HI, Molina A, et al a Phase-3, Multicentered, Double-Blind, Randomized, Placebo-controlled Trial . COU-AA-301. The Lancet Oncology. 2012;13(10):983-992
53- Scher HI, Karim Fizazi, Saad F, et al , a Phase-3, Multicentered, Double-Blind, Randomized, Placebo-controlled Trial. AFFIRM trial, The New England Journal of Medicine. 2012;367(13):1187-1197.
54- Beer TM, Armstrong AJ, Rathkopf DE, et al, a Phase-3, Multicentered, Double-Blind, Randomized, Placebo-controlled Trial. PREVAIL trial , The New England Journal of Medicine. 2014;371(5):424-433
55- Docetaxel based combination therapy for castration resistant prostate cancer .Annals of oncology .2010;2135-2144
56- Loblaw DA, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: the Cancer Care Ontario Practice Guidelines Initiative’s Neuro-Oncology Disease Site Group. J Clin Oncol. 2005;23:2028e 2037.
57- Chow E, Harris K, Fan G, et al. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25:1423e1436
58- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813e822.
59- Khalaf DJ, Annala M, Taavitsainen S, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019;20:1730e 1739
60- Risbridger GP, Taylor RA, Clouston D, et al. Patient-derived xenografts reveal that intraductal carcinoma of the prostate is a prominent pathology in BRCA2 mutation carriers with prostate cancer and correlates with poor prognosis. Eur Urol 2015;67:496-503
61- de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration resistant prostate cancer. N Engl J Med. 2020;382:2091e210
62- Slla A, Konichezky M, Flex D, et al. Low PSA metastatic androgenindependent prostate cancer. Eur Urol 2000;38:250-254.
63- Brennan SM, Gregory DL, Stillie A, et al. Should extrapulmonary small cell cancer be managed like small cell lung cancer? Cancer 2010;116:888-895.
64- Bultijnck R, Van de Caveye I, Rammant E, et al. Clinical pathway improves implementation of evidence-based strategies for the management of androgen deprivation therapy-induced side effects in men with prostate cancer. BJU Int. 2018;121:610e618
- Annexes
Table 1 : Risk stratification according to clinical /Pathologic features
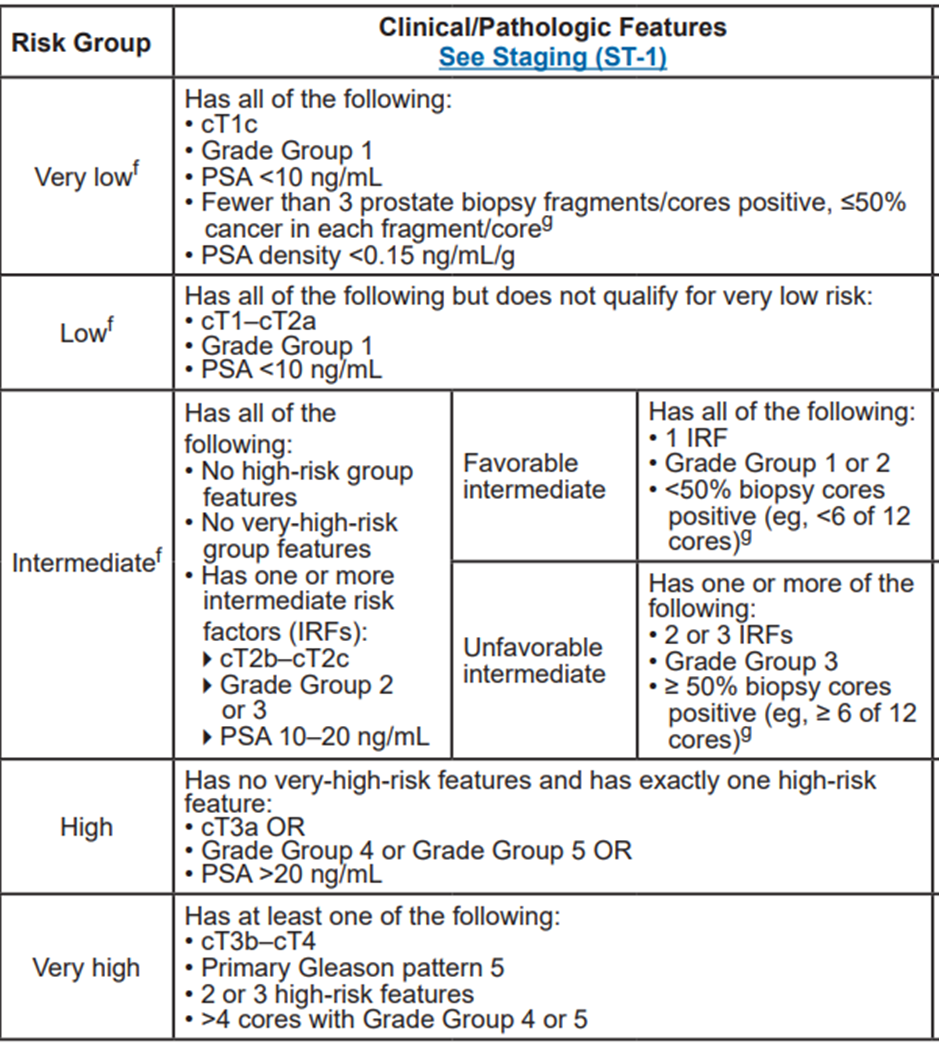
NCCN Clinical Practice Guidelines in Oncology for prostate cancer , version 4.2023
Table 2 : Definitions of active surveillance and watchful waiting
|
Active surveillance |
Watchful waiting |
|
|
Treatment intent |
Curative |
Palliative |
|
Follow-up |
Pre-defined schedule |
Patient-specific |
|
Assessment/markers used |
DRE, PSA, MRI at recruitment, re-biopsy |
Not pre-defined, but dependent on development of symptoms of progression |
|
Life expectancy |
> 10 years |
< 10 years |
|
Aim |
Minimise treatment-related toxicity without compromising survival |
Minimise treatment-related toxicity |
|
Eligible patients |
Mostly low-risk patients |
Can apply to patients with all stages |
EAU Recommendations
Table 3 : AJCC TNM staging system for prostate cancer
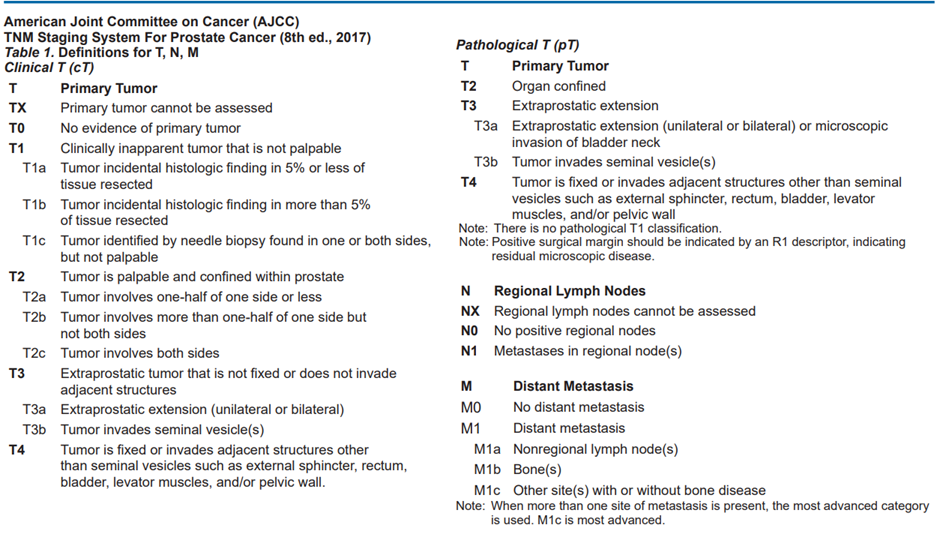
Table 4 : : Definition of Histologic Grade Group (G)
Recently, the Gleason system has been compressed into so-called Grade Groups.
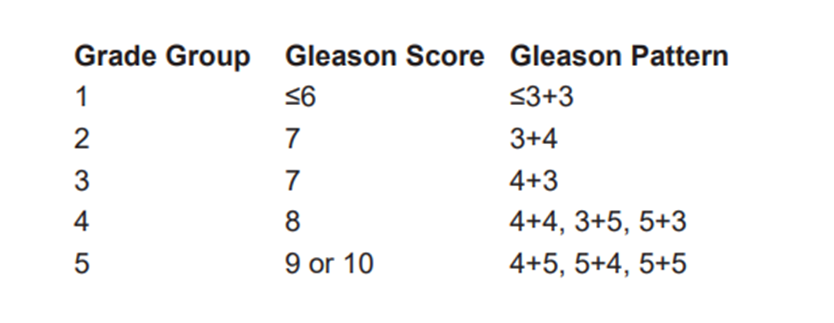
Table 5 : Doses and fractionation of EBRT , Brachytherapy and combined
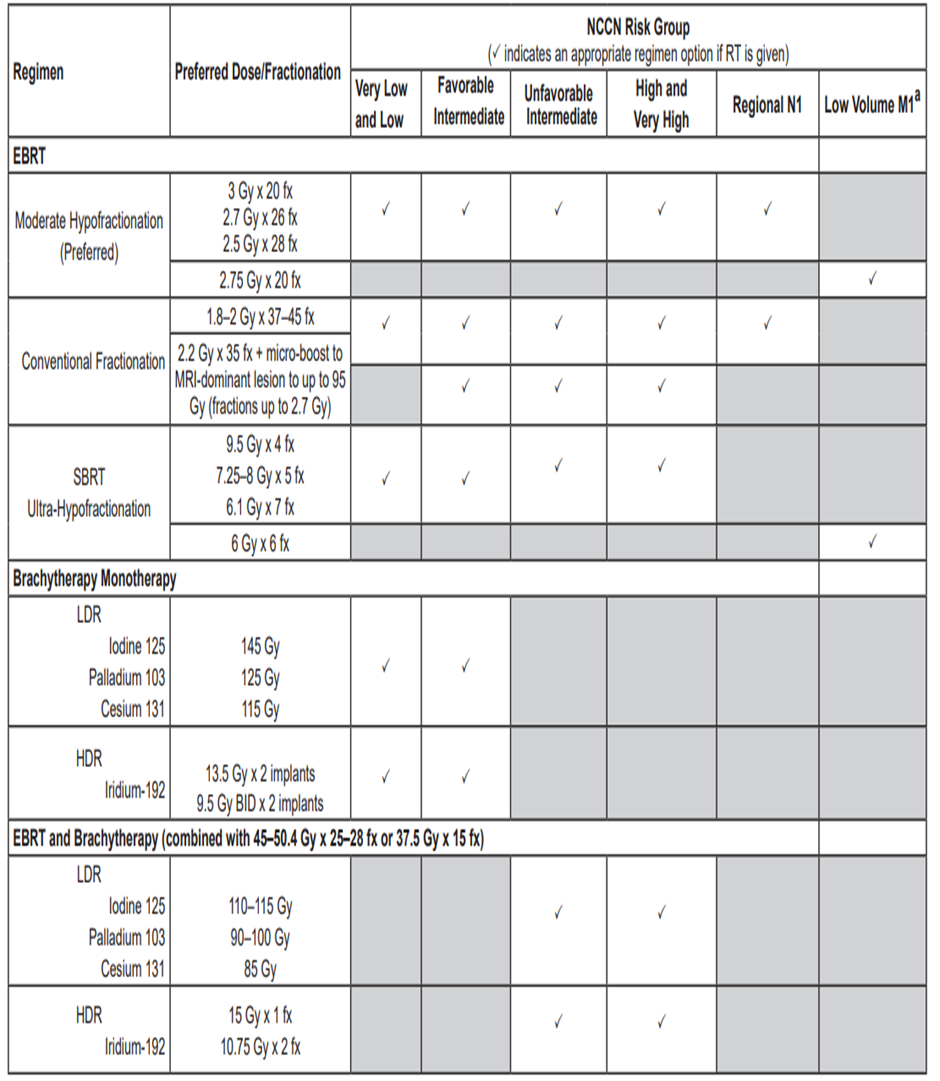
NCCN Clinical Practice Guidelines in Oncology for prostate cancer , version 4.2023