Allergic rhinitis
| Site: | EHC | Egyptian Health Council |
| Course: | Otorhinolaryngology, Audiovestibular & Phoniatrics Guidelines |
| Book: | Allergic rhinitis |
| Printed by: | Guest user |
| Date: | Monday, 23 December 2024, 9:48 PM |
Description
"last update: 28 April 2024"
- Committee
Chair
of the Panel:
Usama Abdel Naseer
➡️Scientific Group Members:
Abdalla Anayet, Abdelrahman Eltahaan, Ahmed Mostafa, Alaa Gaafar, Amr Taha, Ashraf Lotfy, Athar Reda Ibrahim, Bahaa Eltoukhy, Haytham Elfarargy, Hazem Dewidar, Ihab Sifin, Loay Elsharkawy, Mai Mohammed Salama, Mina Esmat, Rania Abdou, Reda Sharkawy, Saad Elzayat, Samir Halim.
➡️Abbreviations
AR: Allergic rhinitis
INC: Intranasal corticosteroid
LTRA: Oral leukotriene receptor antagonist
PAR: Perennial allergic rhinitis
RCT: andomized controlled trial
SAR: Seasonal allergic rhinitis
➡️Glossary
- Allergic rhinitis (AR): Disease caused by an IgE-mediated inflammatory response of the nasal mucous membranes after exposure to inhaled allergens. Symptoms include rhinorrhea (anterior or posterior nasal drainage), nasal congestion, nasal itching, and sneezing.
- Seasonal allergic rhinitis (SAR): Disease caused by an IgE-mediated inflammatory response to seasonal aeroallergens. The length of seasonal exposure to these allergens is dependent on geographic location and climatic conditions.
- Perennial allergic rhinitis (PAR): Disease caused by an IgE-mediated inflammatory response to year-round environmental aeroallergens. These may include dust mites, mold, animal allergens, or certain occupational allergens.
- Intermittent allergic rhinitis: Disease caused by an IgE-mediated inflammatory response and characterized by frequency of exposure or symptoms (<4 days per week or <4 weeks per year).
- Persistent allergic rhinitis: Disease caused by an IgE-mediated inflammatory response and characterized by persistent symptoms (>4 days per week and >4 weeks per year).
- Episodic allergic rhinitis: Disease caused by an IgE-mediated inflammatory response that can occur if an individual is in contact with an exposure that is not normally a part of the individual’s environment. (ie, a cat at a friend’s house).
➡️Scope
This Guideline is concerned with diagnosis and treatment decision of allergic rhinitis, and reduce harmful or unnecessary variations in care. The guideline is intended to be applicable for both pediatric and adult patients with AR. Children under the age of 2 years were excluded because rhinitis in this population may be different than in older patients and is not informed by the same evidence base.
- Executive Summary
▪️ PATIENT HISTORY AND PHYSICAL EXAMINATION: Clinicians should make the clinical diagnosis of AR when patients present with a history and physical examination consistent with an allergic cause and 1 or more of the following symptoms: nasal congestion, runny nose, itchy nose, or sneezing. Findings of AR consistent with an allergic cause include, but are not limited to, clear rhinorrhea, nasal congestion, pale discoloration of the nasal mucosa, and red and watery eyes.
▪️ ALLERGIC TESTING: Clinicians should perform and interpret, or refer to a clinician who can perform and interpret, specific IgE (skin or blood) allergy testing for patients with a clinical diagnosis of AR who do not respond to empiric treatment, or when the diagnosis is uncertain, or when knowledge of the specific causative allergen is needed to target therapy.
▪️ IMAGING: Clinicians should not routinely perform sinonasal imaging in patients presenting with symptoms consistent with a diagnosis of AR.
▪️ ENVIRONMENTAL FACTORS: Clinicians may advise avoidance of known allergens or may advise environmental controls in AR patients who have identified allergens that correlate with clinical symptoms.
▪️ CHRONIC CONDITIONS AND COMORBIDITIES: Clinicians should assess patients with a clinical diagnosis of AR for, and document in the medical record, the presence of associated conditions such as asthma, atopic dermatitis, sleep-disordered breathing, conjunctivitis, rhinosinusitis, and otitis media.
▪️ PHARMACOLOGIC THERAPY:
A- TOPICAL STEROIDS: Clinicians should recommend intranasal steroids for patients with a clinical diagnosis of AR whose symptoms affect their quality of life.
B- ORAL ANTIHISTAMINES: Clinicians should recommend oral second-generation/less sedating antihistamines for patients with AR and primary complaints of sneezing and itching.
C- INTRANASAL ANTIHISTAMINES: Clinicians may offer intranasal antihistamines for patients with seasonal, perennial, or episodic AR.
D- ORAL LEUKOTRIENE RECEPTOR ANTAGONISTS (LTRAs): Clinicians should not offer LTRAs as primary therapy for patients with AR.
E- SALINE NASAL WASH: Saline nasal wash is recommended as part of the treatment strategy for AR.
F- ORAL CORTICOSTEROIDS: Recommendation against the routine use of oral corticosteroids for AR.
G- CROMOLYN: Disodium chromoglycate (DSCG) may be considered for the treatment of AR, particularly in patients known triggers and who cannot tolerate INCSs.
H- INTRANASAL ANTICHOLINERGIC: Ipratropium bromide nasal spray may be considered as an adjunct medication to INCSs in PAR patients with uncontrolled rhinorrhea.
I- OMALIZUMAB: Strong recommendation against use in treatment of allergic rhinitis alone
▪️ COMBINATION THERAPY: Clinicians may offer combination pharmacologic therapy in patients with AR who have inadequate response to pharmacologic monotherapy.
▪️ PHARMACOLOGIC THERAPY OF ALLERGIC RHINITIS ASSOCIATED WITH BRONCHIAL ASTHMA:
✔️ Use of systemic corticosteroid is not recommended for routine use in AR with comorbid asthma.
✔️ Omalizumab: Recommended for those patients with clear IgE-mediated allergic asthma with coexistent AR who fail conventional therapy.
▪️ IMMUNOTHERAPY: Clinicians should offer, or refer to a clinician who can offer, immunotherapy (sublingual or subcutaneous) for patients with AR who have inadequate response to symptoms with pharmacologic therapy.
▪️ INFERIOR TURBINATE REDUCTION: Clinicians may offer, or refer to a surgeon who can offer, inferior turbinate reduction in patients with AR with nasal airway obstruction and enlarged inferior turbinates who have failed medical management.
▪️ HERBAL THERAPY: No recommendation regarding the use of herbal therapy for patients with AR.
- Introduction
Allergic rhinitis (AR) is one of the most common diseases affecting adults and children. It can impair quality of life and, through loss of work and school attendance. Various diagnostic tests and treatments are used in managing patients with this disorder, yet there is considerable variation in their use. This clinical practice guideline was undertaken to optimize the care of patients with AR by addressing quality improvement opportunities through an evaluation of the available evidence and an assessment of the harm-benefit balance of various diagnostic and management options.
➡️Purpose
The primary purpose of this guideline is to address quality improvement opportunities for all clinicians, in any setting, who are likely to manage patients with AR, as well as to optimize patient care, promote effective diagnosis and therapy, and reduce harmful or unnecessary variations in care. The guideline is not intended to replace individualized patient care or clinical judgment.
➡️The target audience
The guideline is intended for all clinicians who are likely to diagnose and manage patients with allergic rhinitis, and it applies to any setting in which allergic rhinitis would be identified, monitored, or managed.
- Methods
A comprehensive search for guidelines was undertaken to identify the most relevant guidelines to consider for adaptation.
inclusion/exclusion criteria followed in the search and retrieval of guidelines to be adapted:
▪️ Selecting only evidence-based guidelines (guideline must include a report on systematic literature searches and explicit links between individual recommendations and their supporting evidence)
▪️ Selecting only national and/or international guidelines
▪️ Specific range of dates for publication (using Guidelines published or updated 2015 and later)
▪️ Selecting peer reviewed publications only
▪️ Selecting guidelines written in English language
▪️ Excluding guidelines written by a single author not on behalf of an organization in order to be valid and comprehensive, a guideline ideally requires multidisciplinary input
▪️ Excluding guidelines published without references as the panel needs to know whether a thorough literature review was conducted and whether current evidence was used in the preparation of the recommendations
The following characteristics of the retrieved guidelines were summarized in a table:
• Developing organisation/authors
• Date of publication, posting, and release
• Country/language of publication
• Date of posting and/or release
• Dates of the search used by the source guideline developers
All retrieved Guidelines were screened and appraised using AGREE II instrument (www.agreetrust.org) by at least two members. the panel decided a cut-off point or rank the guidelines (any guideline scoring above 50% on the rigour dimension was retained)
➡️Evidence assessment
According to WHO handbook for Guidelines we used the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach to assess the quality of a body of evidence, develop and report recommendations. GRADE methods are used by WHO because these represent internationally agreed standards for making transparent recommendations. Detailed information on GRADE is available through the GRC secretariat and on the following sites:
■ GRADE working group: http://www.gradeworkingroup.org
■ GRADE online training modules: http://cebgrade.mcmaster.ca/
■ GRADE profile software: http://ims.cochrane.org/revman/gradepro
Table 1: Quality of evidence in GRADE
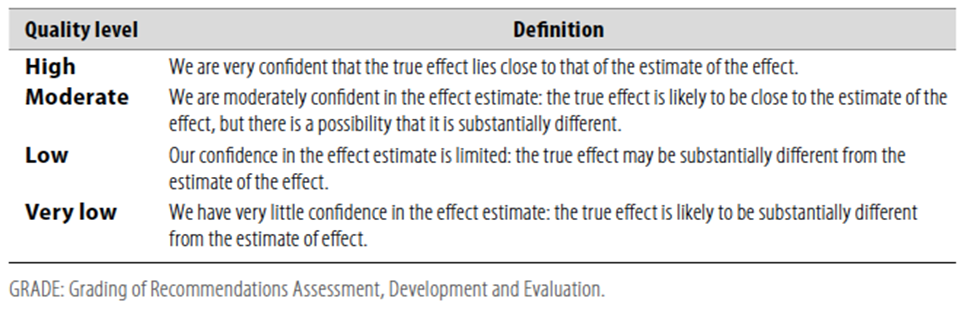
Table 2: Significance of the four levels of evidence
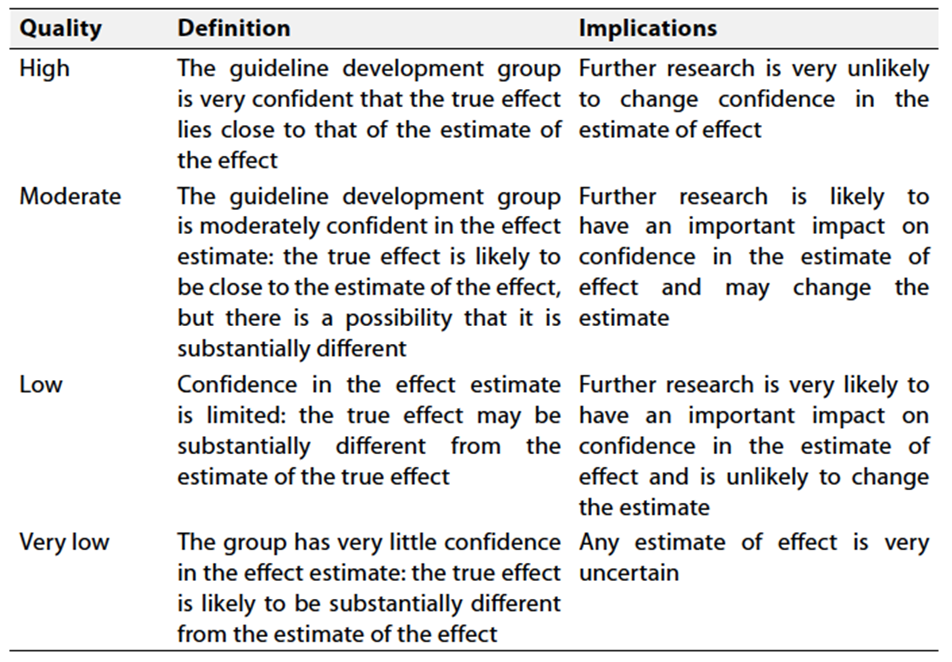
Table 3: Factors that determine How to upgrade or downgrade the quality of evidence

- The strength of the recommendation
The strength of a recommendation communicates the importance of adherence to the recommendation.
➡️Strong recommendations
With strong recommendations, the guideline communicates the message that the desirable effects of adherence to the recommendation outweigh the undesirable effects. This means that in most situations the recommendation can be adopted as policy.
➡️Conditional recommendations
These are made when there is greater uncertainty about the four factors above or if local adaptation has to account for a greater variety in values and preferences, or when resource use makes the intervention suitable for some, but not for other locations. This means that there is a need for substantial debate and involvement of stakeholders before this recommendation can be adopted as policy.
➡️When not to make recommendations
When there is lack of evidence on the effectiveness of an intervention, it may be appropriate not to make a recommendation- Recommendations
1- PATIENT HISTORY AND PHYSICAL EXAMINATION: Clinicians should make the clinical diagnosis of AR when patients present with a history and physical examination consistent with an allergic cause and 1 or more of the following symptoms: nasal congestion, runny nose, itchy nose, or sneezing. Findings of AR consistent with an allergic cause include, but are not limited to, clear rhinorrhea, nasal congestion, pale discoloration of the nasal mucosa, and red and watery eyes.
➡️Strong recommendation
Moderate quality evidence (Observational studies) (3-6)
2- ALLERGY TESTING:
Clinicians should perform and interpret, or refer to a clinician who can perform and interpret, specific IgE (skin or blood) allergy testing for patients with a clinical diagnosis of AR who do not respond to empiric treatment, or when the diagnosis is uncertain, or when knowledge of the specific causative allergen is needed to target therapy.
➡️Strong recommendation
High quality evidence (RCTs and systematic reviews) (7-15)
3- IMAGING:
Clinicians should not routinely perform sinonasal imaging in patients presenting with symptoms consistent with a diagnosis of AR.
➡️Strong recommendation against
Moderate quality evidence (Observational studies) (16,17)
4- ENVIRONMENTAL FACTORS:
Clinicians may advise avoidance of known allergens or
may advise environmental controls (eg, removal of pets;
the use of air filtration systems, bed covers, and acaricides
[chemical agents that kill dust mites]) in AR patients who
have identified allergens that correlate with clinical symptoms.
➡️Conditional recommendation
Moderate quality evidence (Randomized controlled trials and observational studies) (18-23)
5- CHRONIC CONDITIONS AND COMORBIDITIES:
Clinicians should assess patients with a clinical diagnosis of AR for, and document in the medical record, the presence of associated conditions such as asthma, atopic dermatitis, sleep-disordered breathing, conjunctivitis, rhinosinusitis, and otitis media.
➡️Conditional recommendation
Moderate quality evidence (Randomized controlled trials with some heterogeneity) (24-31)
6- PHARMACOLOGIC THERAPY:
A- TOPICAL STEROIDS:
Clinicians should recommend intranasal steroids for patients with a clinical diagnosis of AR whose symptoms affect their quality of life.
➡️Strong recommendation
High quality evidence (RCTs with minor limitations) (32-39)
B- ORAL ANTIHISTAMINES:
Clinicians should recommend oral second-generation/less sedating antihistamines for patients with AR and primary complaints of sneezing and itching.
➡️Strong recommendation
High quality evidence (RCTs with minor limitations) (40-48)
C- INTRANASAL ANTIHISTAMINES:
Clinicians may offer intranasal antihistamines for patients with seasonal, perennial, or episodic AR.
➡️Conditional recommendation
High quality evidence (RCTs and observational studies) (49-53)
D- ORAL LEUKOTRIENE RECEPTOR ANTAGONISTS (LTRAs):
Clinicians should not offer LTRAs as primary therapy for patients with AR.
➡️Strong recommendation against
High quality evidence (RCTs and systematic reviews) (54-57)
E- SALINE NASAL WASH:
Saline nasal wash is recommended as part of the treatment strategy for AR.
➡️Strong recommendation
High quality evidence (RCTs and systematic reviews) (58-64)
F- ORAL CORTICOSTEROIDS :
Recommendation against the routine use of oral corticosteroids for AR. Although not recommended for routine use in AR, certain clinical scenarios warrant the use of short courses of systemic corticosteroids after a discussion of the risks and benefits with the patient. This may include patients with significant nasal obstruction that would preclude penetration of intranasal agents (INCSs or antihistamines). In these cases, a short course of systemic oral corticosteroids could improve congestion and facilitate access and efficacy of the topical agents.
➡️Conditional recommendation against
Moderate quality evidence (RCTs) (65-68)
G- CROMOLYN :
Disodium chromoglycate (DSCG) may be considered for the treatment of AR, particularly in patients known triggers and who cannot tolerate INCSs.
➡️Conditional recommendation
Low quality evidence (69-72)
H- INTRANASAL ANTICHOLINERGIC :
Ipratropium bromide nasal spray may be considered as an adjunct medication to INCSs in PAR patients with uncontrolled rhinorrhea.
➡️Conditional recommendation
Low quality evidence (73-75)
I- OMALIZUMAB:Stronge recommendation against the use of Omalizumab as monotherapy in the treatment of AR. Omalizumab may be used for patients with clear IgE mediated asthma with cpexistent allergic rhinitis who fail conventional therapy. Omalizumab is not currently approved by the FDA for AR treatment. Also as an expensive treatment, cost should be taken into consideration.
➡️Strong recommendation against use in treatment of allergic rhinitis alone
High quality evidence (RCTs and systematic reviews)(76-78)
7- COMBINATION THERAPY:
Clinicians may offer combination pharmacologic therapy in patients with AR who have inadequate response to pharmacologic monotherapy.
➡️Conditional recommendation
Moderate quality evidence (RCTs and observational studies)
There is strong evidence supporting the use of some combinations and the ineffectiveness of other combinations:
▪️ Intranasal Steroids and Intranasal Antihistamines
The combination of INS and intranasal antihistamine is more
effective than INS or intranasal antihistamine monotherapy
for AR. (79-81)
▪️ Intranasal Steroids and Oral Antihistamines:
When patients have no response to INS or incomplete control of nasal symptoms with an INS, oral antihistamines should not be routinely used as additive therapy. (82-84)
▪️ Oral Antihistamines and Oral Decongestants:
Oral antihistamines and oral decongestant combinations control AR symptoms better than either oral antihistamine or oral decongestant alone. Oral decongestant use is not recommended for patients under 4 years of age, and the extended release, 120-mg, 12-hour dose is not recommended for patients under 12 years of age. There is recommendation against long-term use given the significant side effect profile of oral decongestants. (85-88)
▪️ Oral Antihistamines and Leukotriene Receptor Antagonists:
There is conflicting evidence as to whether combined treatment with oral antihistamine and LTRA is superior to either as single treatment, and therefore routine use of combined therapy is not recommended. Combination of oral antihistamine and LTRA is either inferior to or less likely equivalent to INS monotherapy in control of AR symptoms.
Combination therapy with LTRA and oral antihistamine is an option for management of AR, particularly in patients with comorbid asthma or those who do not tolerate INCSs and symptoms are not well-controlled on oral antihistamine monotherapy. (89-95)
▪️ Intranasal Steroids and Leukotriene Receptor Antagonists:
LTRAs should not routinely be used as additive therapy for
patients benefiting from INS for AR. (96-100)
▪️ Intranasal Steroids and Intranasal Oxymetazoline:
The combination of INS and intranasal oxymetazoline is more
effective in controlling AR symptoms than either monotherapy. Short-term use (<3 days) of this combination in cases of severe nasal congestion is recommended. (101-103)
8- PHARMACOLOGIC THERAPY OF ALLERGIC RHINITIS ASSOCIATED WITH BRONCHIAL ASTHMA:
Asthma association with AR and nonallergic rhinitis: Most patients with asthma also have rhinitis, and 10%-40% of rhinitis patients have asthma. IgE mediated inflammation may involve both the upper and lower airways, supporting the unified airway concept.
Rhinitis as a risk factor for asthma: Rhinitis, both allergic and nonallergic, is a risk factor for developing asthma. Asthma and AR also share common risk factors, such as allergen sensitization.
Pharmacotherapy: was reviewed in the treatment of AR with coexisting asthma. Recommendations are as follows:
▪️ Use of pharmacotherapy other than systemic steroids: Recommended for optimal control of AR, with potential additional benefit for coexistent asthma, although not recommended for primary intent of asthma treatment.
▪️ Use of systemic corticosteroid is not recommended for routine use in AR with comorbid asthma due to an unfavorable risk-benefit profile, although certain situations may indicate a short course (eg, acute asthma exacerbation).
▪️ Omalizumab: Recommended for those patients with clear IgE-mediated allergic asthma with coexistent AR who fail conventional therapy. The significant additional cost of this therapy should be considered in its evaluation.
➡️Strong recommendation
High quality evidence (RCTs and Systematic reviews) (104-106)
9- IMMUNOTHERAPY:
Clinicians should offer, or refer to a clinician who can offer, immunotherapy (sublingual or subcutaneous) for patients with AR who have inadequate response to symptoms with pharmacologic therapy.
➡️Strong recommendation
High quality evidence (RCTs and observational studies) (107-113)
10- INFERIOR TURBINATE REDUCTION:
Clinicians may offer, or refer to a surgeon who can offer, inferior turbinate reduction in patients with AR with nasal airway obstruction and enlarged inferior turbinates who have failed medical management.
➡️Conditional recommendation
Moderate quality evidence (Observational studies) (114-117)
11- HERBAL THERAPY:
No recommendation regarding the use of herbal therapy for patients with AR, based on limited knowledge of herbal medicines and concern about the quality of standardization
and safety.
➡️No recommendation
Low quality evidence (118-125)- Clinical Indicators for Monitoring
1- Allergic testing:
Clinicians should perform and interpret, or refer to a clinician who can perform and interpret, specific IgE (skin or blood) allergy testing for patients with a clinical diagnosis of AR who do not respond to empiric treatment, or when the diagnosis is uncertain, or when knowledge of the specific causative allergen is needed to target therapy.
2- Imaging:
Clinicians should not routinely perform sinonasal imaging in patients presenting with symptoms consistent with a diagnosis of AR.
3- Comorbidities:
Clinicians should assess patients with a clinical diagnosis of AR for, and document in the medical record, the presence of associated conditions such as asthma, atopic dermatitis, sleep-disordered breathing, conjunctivitis, rhinosinusitis, and otitis media.
4- Pharmacologic therapy:
Clinicians should not recommend the routine use of oral corticosteroids for AR, and should not use Omalizumab in treatment of allergic rhinitis alone.
These indicators cover aspects such as documentation, diagnostic procedures, treatment decisions, and patient education, providing a comprehensive approach to monitoring physician adherence to the clinical guidelines.
➡️Updating the guideline
To keep these recommendations up to date and ensure its validity it will be periodically updated. This will be done whenever a strong new evidence is available and necessitates updation.
➡️Research Needs
1- Controlled trials are needed comparing surgical versus medical management of inferior turbinate hypertrophy with nasal congestion in patients with AR. In addition, there is a need for further research regarding the role of septoplasty in the treatment of AR.
2- Research is needed to determine the relationship between AR and comorbid conditions such as otitis media and sinusitis. In addition, research is needed to determine the effect of AR treatment on comorbid conditions and the effect of treatment for comorbid conditions on AR.
3- More research, including basic and/or translational trials, is needed to evaluate novel forms of immunotherapy such as peptide vaccines, DNA conjugated vaccines, intradermal injections, and intralymphatic injections. These are all strategies that are hypothesized to reduce the allergenicity of extracts while maintaining or enhancing the beneficial effect on the immune system.
- References
1- Seidman MD, Gurgel RK, Lin SY, et al. AAO-HNSF. Clinical practice guideline: Allergic rhinitis. Otolaryngol Head Neck Surg. 2015 Feb;152(1 Suppl):S1-43.
2- Wise SK, Lin SY, Toskala E. International consensus statement on allergy and rhinology: allergic rhinitis-executive summary. Int Forum Allergy Rhinol. 2018 Feb;8(2):85-107.
3- de Groot H, Brand PL, Fokkens WF, et al. Allergic rhinoconjunctivitis in children. BMJ. 2007;335(7627):985-988.
4- McCrory DC, Williams JW, Dolor RJ, et al. Management of allergic rhinitis in the working-age population. Evid Rep Technol Assess (Summ). 2003;(67):1-4.
5- Varghese M, Glaum MC, Lockey RF. Drug-induced rhinitis. Clin Exp Allergy. 2010;40(3):381-384. Dold S, Wjst M, von Mutius E, et al. Genetic risk for asthma, allergic rhinitis and atopic dermatitis. Arch Dis Child. 1992;67:1018.
6- Wang DY. Risk factors of allergic rhinitis: genetic or environmental? Ther Clin Risk Manag. 2005;1(2):115.
7- Bernstein LE, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(3 suppl 3):S1-S148.
8- Anon JB. Introduction to in vivo allergy testing. Otolaryngol Head Neck Surg. 1993;109(3 pt 2):593-600.
9- 40. Kim BJ, Mun SK. Objective measurements using the skin prick test in allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2010;136(11):1104-1106.
10- Oppenheimer J, Nelson HS. Skin testing: a survey of allergists. Ann Allergy Asthma Immunol. 2006;96(1):19-23.
11- Bousquet J, Heinzerling L, Bachert C, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012;67(1):18-24.
12- Fornadley J, Corey J, Osguthorpe J, et al. Allergic rhinitis: clinical practice guideline. Otolaryngol Head Neck Surg. 1996;115(1):115-122.
13- Krouse J, Mabry R. Skin testing for inhalant allergy 2003: current strategies. Otolaryngol Head Neck Surg. 2003;129(4 suppl):S33- S49.
14- Sicherer SH, Wood RA. Allergy testing in childhood: using allergen-specific IgE tests. Pediatrics. 2012;129(1):193-197.
15- Menardo JL, Bousquet J, Rodiere M, et al. Skin test reactivity in infancy. J Allergy Clin Immunol. 1985;75(6):646-651.
16- Pearce MS, Salotti JA, Little MP, et al. Radiation exposure fromCT scans in childhood and subsequent risk of leukaemia and brain tumours. Lancet. 2012;380(9840):499-505.
17- Katayama H, Yamaguchi K, Kozuka T, et al. Adverse reactions to ionic and nonionic contrast media. Radiology. 1990;175:621-628.
18- Sheikh A, Hurwitz B, Nurmatov U, et al. House dust mite avoidance measures for perennial allergic rhinitis. Cochrane Database Syst Rev. 2010;(7):CD001563.
19- Sheikh A, Hurwitz B. House dust mite avoidance measures for perennial allergic rhinitis. Cochrane Database Syst Rev. 2001;(4):CD001563.
20- Sheikh A, Hurwitz B. House dust mite avoidance measures for perennial allergic rhinitis: a systematic review of efficacy. Br J Gen Pract. 2003;53(489):318-322.
21- Sheikh A, Hurwitz B, Shehata VA. House dust mite avoidance measures for perennial allergic rhinitis. Cochrane Database Syst Rev. 2007;(1):CD001563.
22- Nurmatov U, van Schayk CP, Hurwitz B, et al. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67:158-165.
23- Terreehorst I, Hak E, Oosting AJ, et al. Evaluation of impermeable covers for bedding in patients with allergic rhinitis. N Engl J Med. 2003;349:237-246.
24- Guagris S, Sazonov-Kocevar V, Thomas M. Burden of concomitant allergic rhinitis in adults with asthma. J Asthma. 2006;43(1):1-7.
25- Kapsali T, Horowitz E, Diemer F, et al. Rhinitis is ubiquitous in allergic asthmatics. J Allergy Clin Immunol. 1997;99:S138.
26- Erickson J, Berg A, Lotvall J, et al. Rhinitis phenotypes correlate with different symptom presentation and risk factor patterns of asthma. Respir Med. 2011;105:1611-1621.
27- Boulay ME, Morin A, Laprise C, et al. Asthma and rhinitis: what is the relationship? Curr Opin Allergy Clin Immunol. 2012;12:449-454.
28- Martin PE, Matheson MC, Gurrin L, et al. Childhood eczema and rhinitis predict atopic but not nonatopic asthma: a prospective cohort study over 4 decades. J Allergy Clin Immunol. 2011;127(6):1473-1479.
29- Zeiger RS, Heller S. The development and risk of allergy in high risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol. 1995;95(6):1179-1190.
30- Ricci G, Patrizi A, Baldi E, et al. Long term follow-up of atopic dermatitis: retrospective analysis of related risk factors and association with concomitant allergic diseases. J Am Acad Dermatol. 2006;55(5):765-771.
31- Spergel JM. From atopic dermatitis to asthma, the atopic march. Ann Allergy Asthma Immunol. 2010;105(2):99-106.
32- Bascom R, Wachs M, Naclerio RM, et al. Basophil influx occurs after nasal antigen challenge: effects of topical corticosteroid pretreatment. J Allergy Clin Immunol. 1988;81:580.
33- Pipkorn U, Proud D, Lichtenstein LM, et al. Inhibition of mediator release in allergic rhinitis by pretreatment with topical glucocorticosteroids. N Engl J Med. 1987;316:1506.
34- Erin EM, Leaker BR, Zacharasiewicz AS, et al. Single dose topical corticosteroid inhibits IL-5 and IL13 in nasal lavage following grass pollen challenge. Allergy. 2005;60:1524-1529.
35- Christodoulopoulos P, Cameron L, Durham S, et al. Molecular pathology of allergic disease, II: upper airway disease. J Allergy Clin Immunol. 2000;105:211.
36- Meltzer EO, Jalowayski AA, Orgel A, et al. Subjective and objective assessments in patients with seasonal allergic rhinitis: effects of therapy with mometasone furoate nasal spray. J Allergy Clin Immunol. 1998;102:39-49.
37- Pipkorn U, Proud D, Lichtenstein LM, et al. Effect of short-term systemic glucocorticoid treatment on human nasal mediator release after antigen challenge. J Clin Invest. 1987;80(4):957-961.
38- Baroody FM, Cruz AA, Lichtenstein LM, et al. Intranasal beclomethasone inhibits antigen-induced nasal hyperresponsiveness to histamine. J Allergy Clin Immunol. 1992;90:373.
39- Meyer P, Andersson M, Persson CG, et al. Steroid-sensitive indices of airway inflammation in children with seasonal allergic rhinitis. Pediatr Allergy Immunol. 2003;14:60-65.
40- Mosges R, Konig V, Koberlein J. The effectiveness of modern antihistamines for treatment of allergic rhinitis—an IPD metaanalysis of 140,853 patients. Allergol Int. 2013;62:215-222.
41- Tzanetos DB, Fahrenhol JM, Scott T, et al. Comparison of the sedating effects of levocetirizine and cetirizine: a randomized, double-blind, placebo-controlled trial. Ann Allergy Asthma Immunol. 2011;107(6):517-522.
42- Casale TB, Blaiss MS, Gelfand E, et al. First do no harm: managing antihistamine impairment in patients with allergic rhinitis. J Allergy Clin Immunol. 2003;111(5):S835-S842.
43- Day JH, Briscoe MP, Welsh A. Onset of action, efficacy, and safety of a single dose of fexofenadine hydrochloride for ragweed allergy using an environmental exposure unit. Ann Allergy Asthma Immunol. 1997;79(6):533-540.
44- Kaiser HB, Goplan G, Chung W. Loratadine provides early symptom control in seasonal allergic rhinitis. Allergy Asthma Proc. 2008;29(6):654-658.
45- Prenner BM, Capano D, Harris AG. Efficacy and tolerability of loratadine versus fexofenadine in the treatment of seasonal allergic rhinitis: a double-blind comparison with crossover treatment of nonresponders. Clin Ther. 2000;22(6):760-769.
46- Carlsen KH, Kramer J, Fagertun HE, et al. Loratadine and terfenadine in perennial allergic rhinitis: treatment of nonresponders to the one drug with the other drug. Allergy. 1993;48(6):431-436.
47- Ciprandi G, Passalacqua G, Mincarini M, et al. Continuous versus on demand treatment with cetirizine for allergic rhinitis. Ann Allergy Asthma Immunol. 1997;79(6):507-511.
48- Laekeman G, Simoens S, Buffels J, et al. Continuous versus on-demand pharmacotherapy of allergic rhinitis: evidence and practice. Respir Med. 2010;104(5):615-625.
49- Horak F, Zieglmayer UP, Zieglmayer R, et al. Azelastine nasal spray and Desloratadine tablets in pollen-induced seasonal allergic rhinitis: a pharmacodynamic study of onset of action and efficacy. Curr Med Res Opin. 2006;22:151-157.
50- Kaliner MA, Berger WE, Ratner PH, et al. The efficacy of intranasal antihistamines in the treatment of allergic rhinitis. Ann Allergy Asthma Immunol. 2011;106:S6-S11.
51- LaForce CF, Corren J, Wheeler WJ, et al. Efficacy of Azelastine nasal spray in seasonal allergic rhinitis patients who remain symptomatic after treatment with Fexofenadine. Ann Allergy Asthma Immunol. 2004;93:154-159.
52- Berger WE, White MV; Rhinitis Study Group. Efficacy of Azelastine nasal spray in patients with an unsatisfactory response to loratadine. Ann Allergy Asthma Immunol. 2003;91:205-211.
53- Ratner PH, Findlay SR, Hampel F Jr, et al. A double-blind, controlled trial to assess the safety and efficacy of azelastine nasal spray in seasonal allergic rhinitis. J Allergy Clin Immunol.
54- Gonyeau MJ, Partisano AM. A clinical review of montelukast in the treatment of seasonal allergic rhinitis. Formulary. 2003;38:368-378.
55- Grainger J, Drake-Lee A. Montelukast in allergic rhinitis: a systematic review and meta-analysis. Clin Otolaryngol. 2006;31:360-367.
56- Rodrigo GJ, Yanez A. The role of antileukotriene therapy in seasonal allergic rhinitis: a systematic review of randomized trials. Ann Allergy Asthma Immunol. 2006;96:779-786.
57- Nayak A, Langdon RB. Montelukast in the treatment of allergic rhinitis: an evidence-based review. Drugs. 2007;67:887-901.
58- Cordray S, Harjo JB, Miner L. Comparison of intranasal hypertonic dead sea saline spray and intranasal aqueous triamcinolone spray in seasonal allergic rhinitis. Ear Nose Throat J. 2005; 84: 426–430.
59- Rogkakou A, Guerra L, Massacane P, et al. Effects on symptoms and quality of life of hypertonic saline nasal spray added to antihistamine in persistent allergic rhinitis—a randomized controlled study. Eur Ann Allergy Clin Immunol. 2005; 37: 353–356.
60- Ural A, Oktemer TK, Kizil Y, Ileri F, Uslu S. Impact of isotonic and hypertonic saline solutions on mucociliary activity in various nasal pathologies: clinical study. J Laryngol Otol. 2009; 123: 517–521.
61- Chusakul S, Warathanasin S, Suksangpanya N, et al. Comparison of buffered and nonbuffered nasal saline irrigations in treating allergic rhinitis. Laryngoscope. 2013; 123: 53–56.
62- Garavello W, Romagnoli M, Sordo L, Gaini RM, Di Berardino C, Angrisano A. Hypersaline nasal irrigation in children with symptomatic seasonal allergic rhinitis: a randomized study. Pediatr Allergy Immunol. 2003; 14: 140–143.
63- Garavello W, Di Berardino F, Romagnoli M, Sambataro G, Gaini RM. Nasal rinsing with hypertonic solution: an adjunctive treatment for pediatric seasonal allergic rhinoconjunctivitis. Int Arch Allergy Immunol. 2005; 137: 310–314.
64- Hermelingmeier KE, Weber RK, Hellmich M, Heubach CP, Mosges R. Nasal irrigation as an adjunctive treatment in allergic rhinitis: a systematic review and meta-analysis. Am J Rhinol Allergy. 2012; 26: e119–e125.
65- Schwartz E. Oral hydrocortisone therapy in bronchial asthma and bay fever. J Allergy. 1954; 25: 112–119.
66- Brooks CD, Karl KJ, Francom SF. Oral methylprednisolone acetate (Medrol Tablets) for seasonal rhinitis: examination of dose and symptom response. J Clin Pharmacol. 1993; 33: 816–822.
67- Kwaselow A, McLean J, Busse W, et al. A comparison of intranasal and oral flunisolide in the therapy of allergic rhinitis. Evidence for a topical effect. Allergy. 1985; 40: 363–367.
68- Karaki M, Akiyama K, Mori N. Efficacy of intranasal steroid spray (mometasone furoate) on treatment of patients with seasonal allergic rhinitis: comparison with oral corticosteroids. Auris Nasus Larynx. 2013; 40: 277–281.
69- Lejeune M, Lefebvre PP, Delvenne P, El-Shazly AE. Nasal sodium cromoglycate (Lomusol) modulates the early phase reaction of mild to moderate persistent allergic rhinitis in patients mono-sensitized to house dust mite: a preliminary study. Int Immunopharmacol. 2015; 26: 272–276.
70- Tandon MK, Strahan EG. Double-blind crossover trial comparing beclomethasone dipropionate and sodium cromoglycate in perennial allergic rhinitis. Clin Allergy. 1980; 10: 459–462.
71- McDowell MK, Spitz E. Treatment of chronic perennial allergic rhinitis: a double-blind trial of cromolyn sodium. Ann Allergy. 1977; 39: 169–174.
72- Warland A, Kapstad B. The effect of disodium cromoglycate in perennial allergic rhinitis. A controlled clinical study. Acta Allergol. 1977; 32: 195–199.
73- Kim KT, Kerwin E, Landwehr L, et al. Use of 0.06% ipratropium bromide nasal spray in children aged 2 to 5 years with rhinorrhea due to a common cold or allergies. Ann Allergy Asthma Immunol. 2005; 94: 73–79.
74- Ensing K, de Zeeuw RA, Nossent GD, Koeter GH, Cornelissen PJ. Pharmacokinetics of ipratropium bromide after single dose inhalation and oral and intravenous administration. Eur J Clin Pharmacol. 1989; 36: 189–194.
75- Dockhorn R, Aaronson D, Bronsky E, et al. Ipratropium bromide nasal spray 0.03% and beclomethasone nasal spray alone and in combination for the treatment of rhinorrhea in perennial rhinitis. Ann Allergy Asthma Immunol. 1999; 82: 349–359.
76- Kopp MV, Hamelmann E, Zielen S, et al. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co-morbid seasonal allergic asthma. Clin Exp Allergy. 2009; 39: 271–279.
77- Kopp MV, Hamelmann E, Bendiks M, et al. Transient impact of omalizumab in pollen allergic patients undergoing specific immunotherapy. Pediatr Allergy Immunol. 2013; 24: 427–433.
78- Chaker AM, Shamji MH, Dumitru FA, et al. Short-term subcutaneous grass pollen immunotherapy under the umbrella of anti-IL-4: A randomized controlled trial. J Allergy Clin Immunol. 2016; 137: 452–461.e9.
79- Badorrek P, Dick M, Schauerte A, et al. A combination of cetirizine and pseudoephedrine has therapeutic benefits when compared to single drug treatment in allergic rhinitis. Int J Clin Pharmacol Ther. 2009;47(2):71-77.
80- Meltzer EO, LaForce C, Ratner P, et al. MP29-02 (a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate) in the treatment of seasonal allergic rhinitis: a randomized, double-blind, placebo-controlled trial of efficacy and safety. Allergy Asthma Proc. 2012;33(4):324-332.
81- Carr W, Bernstein JP, Lieberman P, et al. A novel intranasal therapy of azelastine with fluticasone for the treatment of allergic rhinitis. J Allergy Clin Immunol. 2012;129:1282-1289.
82- Anolik R. Clinical benefits of combination treatment with mometasone furoate nasal spray and loratadine vs monotherapy with mometasone furoate in the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2008;100(3):264-271.
83- Benincasa C, Lloyd RS. Evaluation of fluticasone propionate aqueous nasal spray taken alone and in combination with cetirizine in the prophylactic treatment of seasonal allergic rhinitis. Drug Investig. 1994;8:225-233.
84- Nasser M, Fedorowicz Z, Aljufairi H, et al. Antihistamines used in addition to topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2010;(7):CD006989.
85- Schenkel E, Corren J, Murray JJ. Efficacy of once-daily desloratadine/ pseudoephedrine for relief of nasal congestion. Allergy Asthma Proc. 2002;23(5):325-330.
86- Grosclaude M, Mees K, Pinelli ME, et al. Cetirizine and pseudoephedrine retard, given alone or in combination, in patients with seasonal allergic rhinitis. Rhinology. 1997;35(2):67-73.
87- Bronsky E, Boggs P, Findlay S, et al. Comparative efficacy and safety of a once-daily loratadine-pseudoephedrine combination versus its components alone and placebo in the management of seasonal allergic rhinitis. J Allergy Clin Immunol. 1995;96(2):139-147.
88- Berkowitz RB, McCafferty F, Lutz C, et al. Onset of action of fexofenadine hydrochloride 60 mg/pseudoephedrine hydrochloride 120 mg in subjects aged 12 years with moderate to severe seasonal allergic rhinitis: a pooled analysis of two single-dose, randomized, double-blind, placebo-controlled allergen exposure unit studies. Clin Ther. 2006;28(10):1658-1669.
89- Lu S, Malice MP, Dass SB, et al. Clinical studies of combination montelukast and loratadine in patients with seasonal allergic rhinitis. J Asthma. 2009;46(9):878-883.
90- Ciebiada M, Barylski M, Gorska Ciebiada M. Nasal eosinophilia and serum soluble intercellular adhesion molecule 1 in patients with allergic rhinitis treated with montelukast alone or in combination with desloratadine or levocetirizine. Am J Rhinol Allergy. 2013;27(2):e58-e62.
91- Watanasomsiri A, Poachanukoon O, Vichyanond P. Efficacy of montelukast and loratadine as treatment for allergic rhinitis in children. Asian Pac J Allergy Immunol. 2008;26(2-3):89-95.
92- Nayak AS, Philip G, Lu S, et al. Montelukast Fall Rhinitis Investigator G. Efficacy and tolerability of montelukast alone or in combination with loratadine in seasonal allergic rhinitis: a multicenter, randomized, double-blind, placebo-controlled trial performed in the fall. Ann Allergy Asthma Immunol. 2002;88(6):592-600.
93- Wilson AM, Orr LC, Sims EJ, et al. Effects of monotherapy with intra-nasal corticosteroid or combined oral histamine and leukotriene receptor antagonists in seasonal allergic rhinitis. Clin Exp Allergy. 2001;31:61-68.
94- Lombardo G, Quattrocchi P, Lombardo GR, et al. Concomitant levocetirizine and montelukast in the treatment of seasonal allergic rhinitis: influence on clinical symptoms. Italian Journal of Allergy and Clinical Immunology. 2006;16:63-68.
95- Meltzer EO, Malmstrom K, Lu S, et al. Concomitant montelukast and loratadine as treatment for seasonal allergic rhinitis: a randomized, placebo-controlled clinical trial. J Allergy Clin Immunol. 2000;105:917-922.
96- Di Lorenzo G, Pacor ML, Pellitteri ME, et al. Randomized placebo-controlled trial comparing fluticasone aqueous nasal spray in mono-therapy, fluticasone plus cetirizine, fluticasone plus montelukast and cetirizine plus montelukast for seasonal allergic rhinitis. Clin Exp Allergy. 2004;34(2):259-267.
97- Pullerits T, Praks L, Ristioja V, et al. Comparison of a nasal glucocorticoid, antileukotriene, and a combination of antileukotriene and antihistamine in the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. 2002;109(6):949-955.
98- Saengpanich S, deTineo M, Naclerio RM, et al. Fluticasone nasal spray and the combination of loratadine and montelukast in seasonal allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2003;129(5):557-562.
99- Modgill V, Badyal DK, Verghese A. Efficacy and safety of montelukast add-on therapy in allergic rhinitis. Methods Find Exp Clin Pharmacol. 2010;32(9):669-674.
100- Esteitie R, deTineo M, Naclerio RM, et al. Effect of the addition of montelukast to fluticasone proprionate for the treatment of perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2010;105:155-161.
101- Lau SK, Wei WI, Van Hasselt CA, et al. A clinical comparison of budesonide nasal aerosol, terfenadine and a combined therapy of budesonide and oxymetazoline in adult patients with perennial rhinitis. Asian Pac J Allergy Immunol. 1990;8(2): 109-115.
102- Meltzer EO, Bernstein DI, Prenner BM, et al. Mometasone furoate nasal spray plus oxymetazoline nasal spray: short-term efficacy and safety in seasonal allergic rhinitis. Am J Rhinol Allergy. 2013;27(2):102-108.
103- Baroody FM, Brown D, Gavanescu L, et al. Oxymetazoline adds to the effectiveness of fluticasone furoate in the treatment of perennial allergic rhinitis. J Allergy Clin Immunol. 2011;127:927-934.
104- Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010; 126: 466–476.
105- Bhargava S, Prakash A, Rehan HS, Gupta LK. Effect of systemic corticosteroids on serum apoptotic markers and quality of life in patients with asthma. Allergy Asthma Proc. 2015; 36: 275–282.
106- Vignola AM, Humbert M, Bousquet J, et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy. 2004; 59: 709–717.
107- Lin SY, Erekosima N, Kim JM, et al. Sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review. JAMA. 2013;309(12):1278-1288.
108- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;121:S1-S55.
109- Erekosima N, Suarez-Cuervo C, Ramanathan M, et al. Effectiveness of subcutaneous immunotherapy for allergic rhinoconjunctivitis and asthma: a systematic review. Laryngoscope. 2014;124(3):616-627.
110- Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;(1):CD001936.
111- Matricardi PM, Kuna P, Panetta V, et al. Subcutaneous immunotherapy and pharmacotherapy in seasonal allergic rhinitis: a comparison based on meta-analyses. J Allergy Clin Immunol. 2011;128(4):791-799.
112- Wilson DR, Torres LI, Durham SR. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003;(2):CD002893.
113- Radulovic S, Wilson D, Calderon M, et al. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011;66(6):740-752.
114- Passali D, Passali FM, Damiani V, et al. Treatment of inferior turbinate hypertrophy: a randomized clinical trial. Ann Otol Rhinol Laryngol. 2003;112:683-688.
115- Lee JY, Lee JD. Comparative study on the long-term effectiveness between coblation and microdebrider assisted partial turbinoplasty. Laryngoscope. 2006;116(5):729-734.
116- Gunhan K, Unlu H, Yuceturk AV, et al. Intranasal steroids or radiofrequency turbinoplasty in persistent allergic rhinitis: effects on quality of life and objective parameters. Eur Arch Otorhinolaryngol. 2011;268(6):845-850.
117- Mori S, Fujieda S, Yamada T, et al. Submucous turbinectomy decreases not only nasal stiffness but also sneezing and rhinorrhea in patients with perennial AR. Clin Exp Allergy. 1999;29(11):1542-1548.
118- Chen K, Yu B. Certain progress of clinical research on Chinese integrative medicine. Chin Med J. 1999;112:934-937.
119- Seidman MD, Grinsven GV. Complementary and integrative treatments: integrative care centers and hospitals: one center’s perspective. Otolaryngol Clin North Am. 2013;46(3):485-497.
120- Xue CC, Li CG, Hugel HM, et al. Does acupuncture or Chinese herbal medicine have a role in the treatment of allergic rhinitis? Curr Opin Allergy Clin Immunol. 2006;6:175-179.
121- Xue DD, Hugel HM, Li CG, et al. Efficacy, chemistry and pharmacology of Chinese herbal medicine for allergic rhinitis. Curr Med Chem. 2004;11:1403-1421.
122- Brinkhaus B, Hummelsberger J, Kohnen R, et al. Acupuncture and Chinese herbal medicine in the treatment of patients with seasonal allergic rhinitis: a randomized-controlled clinical trial. Allergy. 2004;59:953-960.
123- Hu G, Walls RS, Bass D, et al. The Chinese herbal formulation biminne in management of perennial allergic rhinitis: a randomized, double-blind, placebo-controlled, 12 week clinical trial. Ann Allergy Asthma Immunol. 2002;88:478-487.
124- Borchers AT, Hackman RM, Keen CL, et al. Complementary medicine: a review of immunomodulatory effects of Chinese herbal medicines. Am J Clin Nutr. 1997;66:1303-1312.
125- Latchman Y, Banerjee P, Poulter LW, et al. Association of immunological changes with clinical efficacy in atopic eczema patients treated with traditional Chinese herbal therapy (Zemaphyte). Int Arch Allergy Immunol. 1996;109:242-249.