Prevention or Delay of Type 2 Diabetes Mellitus
| Site: | EHC | Egyptian Health Council |
| Course: | Diabetes and Endocrinology Guidelines |
| Book: | Prevention or Delay of Type 2 Diabetes Mellitus |
| Printed by: | Guest user |
| Date: | Monday, 23 December 2024, 9:45 PM |
Description
"last update: 14 February 2024"
- Committee
Chair of the GDG: Mohamed Hesham El Hefnawy, National Institute of Diabetes and
Endocrinology, Cairo
Members of the Guideline Development Group (GDG):
Randa Salam, Faculty of Medicine, Cairo University, Cairo
Athar Reda Ibrahim, National Institute of Diabetes and Endocrinology, Cairo
Amr Ali Mahfouz, National Institute of Diabetes and Endocrinology, Cairo
Atef Bassyouni, National Institute of Diabetes and Endocrinology, Cairo
Elsayed Abdel Fattah Eid, Faculty of Medicine, Delta University for science and technology, Dakahlia
Fawzy A F Elmessallamy, Faculty of Medicine, Zagazig University, Sharqia
Mohamed Abdelhady Mohamed Mashahet, Faculty of Medicine, Fayoum University, Fayoum.
Mohamed Abdelmoniem Elmikawy, police hospital, Cairo.
Yara Muhammad Ahmad Eid, Faculty of Medicine, Ain Shams University, Cairo.
Abbreviations
BMI
Body Mass Index
CVD
Cardiovascular disease
DASH diet
Dietary Approach to Stop Hypertension
DPP
Diabetes Prevention Program
GRADE
Grading of Recommendations, Assessment, Development and Evaluation
HbA1c
Glycated hemoglobin A1c
RCT
Randomized controlled trial
➡️Glossary
Cardiovascular diseases (CVDs)
A group of disorders of the heart and blood vessels that include coronary heart disease, cerebrovascular disease and peripheral arterial disease.
HbA1c
Haemoglobin that is glycated by non-enzymatic attachment of glucose to haemoglobin. The concentration of HbA1c is the most commonly used measure of chronic glycaemia in clinical trials and diabetes management. It is used to reflect the integrated mean glucose level over the previous 8–12 weeks.
Metformin
A biguanide oral hypoglycemic agent used in treating type 2 diabetes. Complex mechanism of action
It decreases glucose production by the liver and enhances insulin sensitivity, opposes anti-inflammatory and anti-oxidant effects.
Prediabetes:
Prediabetes is a serious health condition where blood sugar levels are higher than normal, but not high enough yet to be diagnosed as type 2 diabetes.
Type 2 diabetes
A metabolic disease characterized by hyperglycemia, resulting from a defect in insulin secretion, insulin action or both. Long-term hyperglycemia is associated with micro and macrovascular complications.
- Executive Summary
The prevalence of diabetes is globally increasing with a high incidence of complications. Targeted interventions and support are important in this high-risk group. Globally, more than 570 million adults live with diabetes so Prevention or delay of type 2 diabetes Mellitus (T2DM) is of great importance.
This guideline offers evidence-based recommendations for the prevention of diabetes. The recommendations are intended to provide healthcare professionals with practical guidance on preventing or delaying diabetes and associated co-morbidities improving healthy lifestyles for people with high risk of type 2 diabetes.
Recommendations |
|
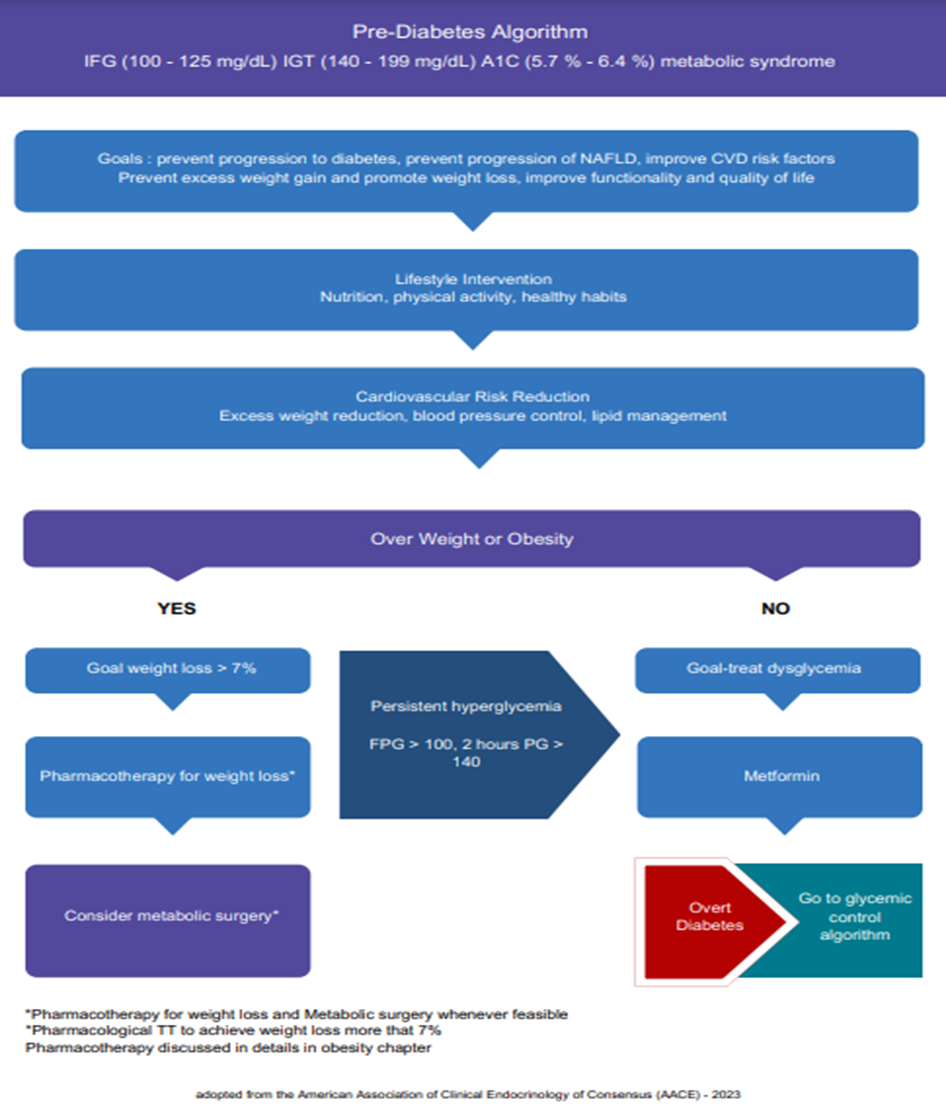
l Refer adults with overweight/obesity at high risk of type 2 diabetes, to an intensive lifestyle behavior change program for a weight reduction of at least 7% of initial body weight through a healthy reduced-calorie diet and 150 min/week of moderate-intensity physical activity. (Strong recommendation). l A variety of eating patterns should be considered to prevent diabetes in individuals with prediabetes. Including Mediterranean diet, low carbohydrate eating plan, low fat, DASH diet (Dietary approaches to stop hypertension) (Strong recommendation) l Prescribe metformin for prevention of T2DM in adult individuals with prediabetes, 25-59 years, those with high BMI ≥35 kg/m2, higher fasting plasma glucose 100 - 125 mg/dL, higher HbA1C 5.7 – 6.4%, women with prior GDM (strong recommendation) l Prediabetes is associated with heightened cardiovascular risk; therefore, screening for and treatment of modifiable risk factors for cardiovascular disease should be considered. (Strong recommendation) l More intensive preventive approaches should be considered in individuals who are at particularly high risk of progression to diabetes, including individuals with BMI ≥35 kg/m2, those at higher glucose levels (e.g., fasting plasma glucose 100 –125 mg/dL, 2-h postprandial glucose 140 – 199 mg/dL, A1C 5.7 – 6.4%), and individuals with a history of gestational diabetes mellitus (strong recommendation) l Pharmacotherapy should be considered to achieve sustained weight loss, minimize the progression of hyperglycemia, and cardiovascular risk reduction. (strong recommendation) |
- Introduction
The prevalence of T2DM is rapidly increasing with substantial personal and economic burden. Delay and prevention of diabetes is considered an important target for various interventions including lifestyle modification which has proven effective in preventing incident diabetes in high risk groups.
Various medications can also prevent or delay diabetes, whether diabetes prevention strategies also ultimately prevent the development of diabetic vascular complications are unknown, but cardiovascular risk factors are favorably affected.
Wide spread application has however been limited by local financial considerations.
➡️Scope and purpose
The purpose of the work is to identify the best clinical practice guidelines in the prevention and delay of diabetes and to create actionable recommendations for healthcare professionals, adults with T2DM, and their families.
The objectives of these guidelines are :
- To guide the proper program regarding lifestyle behavior change for diabetes prevention
-To consider the use of metformin therapy that should be used in adults at high risk for the development of diabetes.
➡️Target audience
This guideline targets, healthcare professionals (internists, endocrinologists, family medicine physicians), dieticians, researchers, policymakers, public health practitioners, national diabetes programme managers, as well as non-governmental organizations (NGO).
- Methodology
A comprehensive search for guidelines was undertaken to identify the most relevant guidelines to consider for adaptation.
Inclusion/ exclusion criteria followed in the search and retrieval of guidelines to be adapted:
• Selecting only evidence-based guidelines (guideline must include a report on systematic literature searches and explicit links between individual recommendations and their supporting evidence)
• Selecting only national and/or international guidelines
• Specific range of dates for publication (using Guidelines published or updated in 2015 and later)
• Selecting peer reviewed publications only
• Selecting guidelines written in English language
• Excluding guidelines written by a single author, not on behalf of an organization to be valid and comprehensive, a guideline ideally requires multidisciplinary input
• Excluding guidelines published without references as the panel needs to know whether a thorough literature review was conducted and whether current evidence was used in the preparation of the recommendations
The following characteristics of the retrieved guidelines were summarized in a table:
• Developing organisation/authors
• Date of publication, posting, and release
• Country/language of publication
• Date of posting and/or release
• Dates of the search used by the source guideline developers
All retrieved Guidelines were screened and appraised using AGREE II instrument (www.agreetrust.org) by at least three members. The panel decided on a cut-off point or ranked the guidelines (any guideline scoring above 50% on the rigor dimension was retained). The GDG decided to adapt the American Diabetes Association – Standards of Care in Diabetes – 2024.
- Evidence assessment
According to WHO Handbook for Guidelines, we used the GRADE (Grading of
Recommendations, Assessment, Development and Evaluation) approach to assess the quality of a body of evidence, develop and report recommendations. GRADE methods are used by WHO because these represent internationally agreed standards for making transparent recommendations. Detailed GRADE information is available on the following sites:
• GRADE working group: http://www.gradeworkingroup.org
• GRADE online training modules: http://cebgrade.mcmaster.ca/
• GRADE profile software: http://ims.cochrane.org/revman/gradepro
Table 1 Quality and Significance of the four levels of evidence in GRADE:
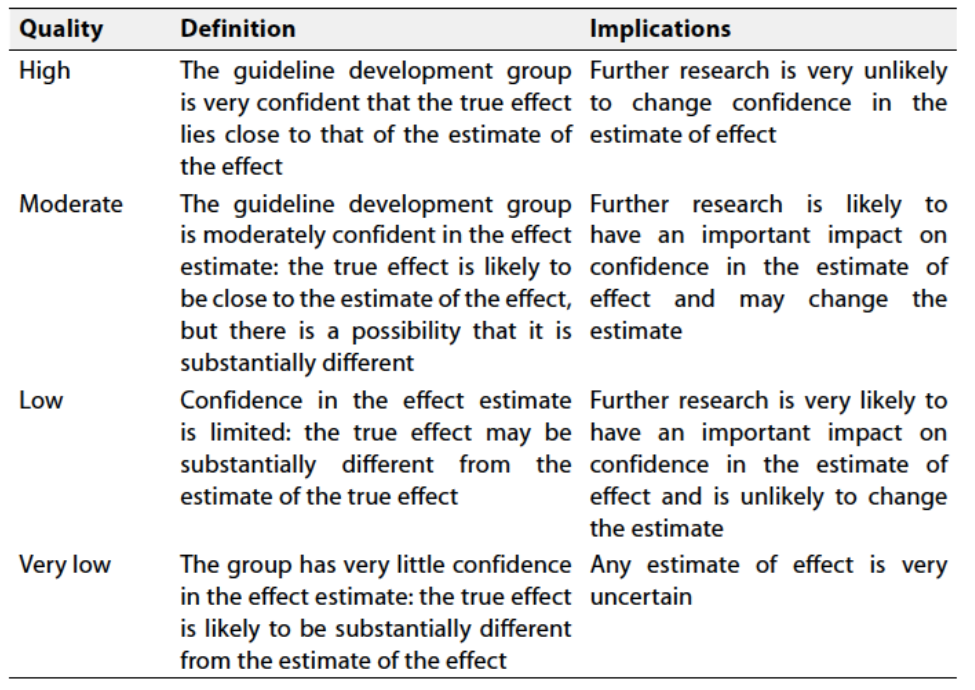
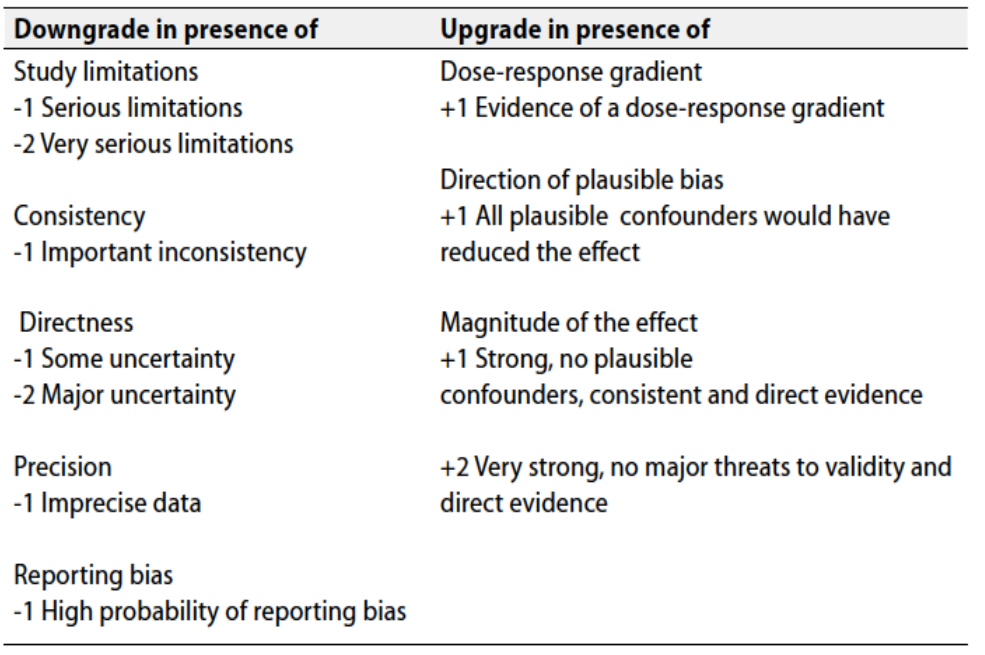
Table 2 Factors that determine How to upgrade or downgrade the quality of evidence
The strength of the recommendation
The strength of a recommendation communicates the importance of adherence to the recommendation.
➡️Strong recommendations
With strong recommendations, the guideline communicates the message that the desirable effects of adherence to the recommendation outweigh the undesirable effects. This means that in most situations the recommendation can be adopted as policy.
➡️Conditional recommendations
These are made when there is greater uncertainty about the four factors above or if local adaptation has to account for a greater variety in values and preferences, or when resource use makes the intervention suitable for some, but not for other locations. This means that there is a need for substantial debate and involvement of stakeholders before this recommendation can be adopted as policy.
➡️When not to make recommendations
When there is lack of evidence on the effectiveness of an intervention, it may be appropriate not to make a recommendation.
- Recommendations
Recommendation
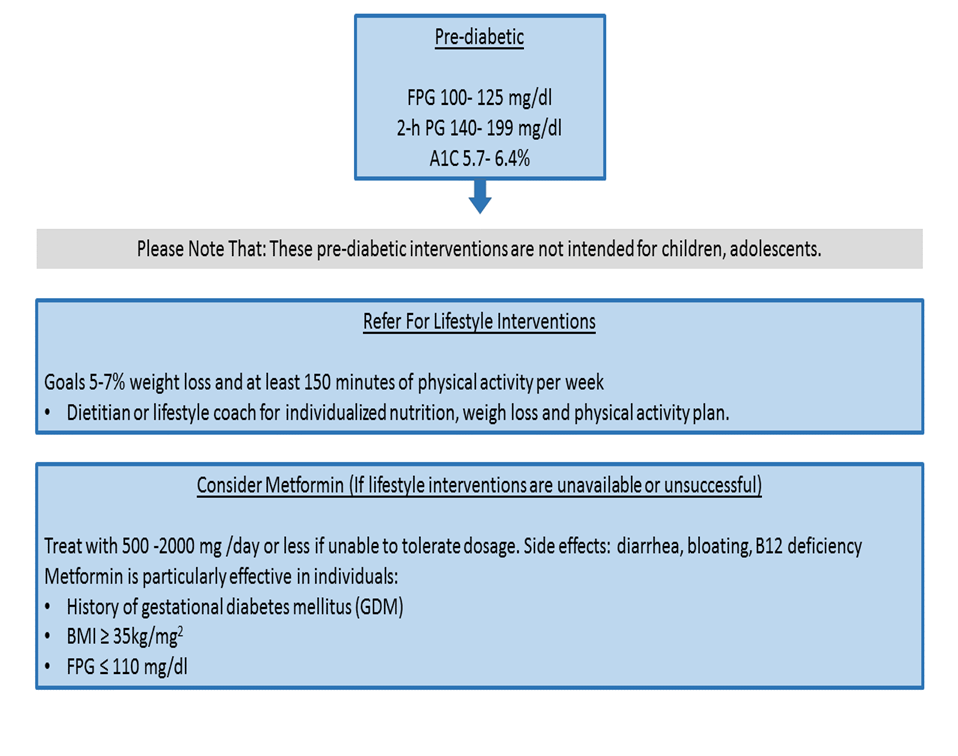
a- Refer adults with overweight/obesity at high risk of type 2 diabetes, to an intensive lifestyle behavior change program for a weight reduction of at least 7% of initial body weight through a healthy reduced-calorie diet and 150 min/week of moderate intensity physical activity. (Strong recommendation, high certainty evidence)
Summary of evidence
Several major randomized controlled trials, including the Diabetes Prevention Program (DPP) trial[i] , the Finnish Diabetes Prevention Study (DPS) [ii] , and the Da Qing Diabetes Prevention Study (Da Qing study) [iii] , demonstrate that lifestyle/ behavioral intervention with an individualized reduced-calorie meal plan is highly effective in preventing or delaying type 2 diabetes and improving other cardio-metabolic markers (such as blood pressure, lipids, and inflammation) [iv] .
The strongest evidence for diabetes prevention in the U.S. comes from the DPP trial1. The DPP demonstrated that intensive lifestyle intervention could reduce the risk of incident type 2 diabetes by 58% over 3 years. Follow-up of three large studies of lifestyle intervention for diabetes prevention showed a sustained reduction in the risk of progression to type 2 diabetes: 39% reduction at 30 years in the Da Qing study[v] , 43% reduction at 7 years in the Finnish DPS 2, and 34% reduction at 10 years[vi] and 27% reduction at 15 years [vii] in the U.S. Diabetes Prevention Program Outcomes Study (DPPOS).
The two major goals of the DPP intensive lifestyle intervention were to achieve and maintain a minimum of 7% weight loss and 150 min moderate-intensity physical activity per week, such as brisk walking.
The DPP lifestyle intervention was a goal-based intervention. All participants were given the same weight loss and physical activity goals, but individualization was permitted in the specific methods used to achieve the goals[viii] . Although weight loss was the most important factor in reducing the risk of incident diabetes, it was also found that achieving the target behavioral goal of at least 150 min of physical activity per week, even without achieving the weight loss goal, reduced the incidence of type 2 diabetes by 44% [ix] .
Rationale for the recommendation
The 7% weight loss goal was selected because it was feasible to achieve and maintain and likely to lessen the risk of developing diabetes. Participants were encouraged to achieve ≥7% weight loss during the first 6 months of the intervention. Further analysis suggests maximal prevention of diabetes with at least 7–10% weight loss9.
The goal for physical activity was selected to approximate at least 700 kcal/ week expenditure from physical activity. For ease of translation, this goal was described as at least 150 mins of moderate intensity physical activity per week, similar in intensity to brisk walking. Participants were encouraged to distribute their activity throughout the week with a minimum frequency of three times per week and at least 10 min per session. A maximum of 75 min of strength training could be applied toward the total 150 min/week physical activity goal8.
Breaking up prolonged sedentary time may also be encouraged, as it is associated with moderately lower postprandial glucose levels[x] ,[xi]. The preventive effects of physical activity appear to extend to the prevention of gestational diabetes mellitus (GDM) [xii] .
b. Lifestyle modifications: Intensive lifestyle modifications can reduce the incidence of T2DM. Lifestyle modifications include a healthy diet, increased physical activity, and encouraged weight loss for overweight or obese individuals.
c. Body weight management and physical activity Recommendations Refer Individuals with prediabetes to an intensive behavioral lifestyle intervention program with a target to achieve and maintain a 7% loss of their initial body weight. Increase moderate-intensity physical activity (such as brisk walking) to at least 150 min/week.
Recommendations
b- A variety of eating patterns can be considered to prevent diabetes in individuals with prediabetes. including Mediterranean diet, low carbohydrate eating plan, low fat, DASH diet (Dietary approaches to stop hypertension ) (Strong recommendation, moderate certainty evidence)
Remarks
Nutrition counseling for weight loss in the DPP lifestyle intervention arm included a reduction of total dietary fat and calories1,8,9. However, evidence suggests that there are not an ideal percentage of calories from carbohydrates, protein, and fat for all people to prevent diabetes; therefore, macronutrient distribution should be based on an individualized assessment of current eating patterns, preferences, and metabolic goals10. Based on other intervention trials, a variety of eating patterns characterized by the totality of food and beverages habitually consumed[xiii] ,[xiv] may also be appropriate for individuals with prediabetes13
Summary of evidence
Mediterranean-style and low-carbohydrate eating plans (high in vegetables, fruits, whole grains, beans, nuts and seeds, and olive oil (. [xv] ,[xvi],[xvii],[xviii]. Observational studies have also shown that vegetarian, plant-based (may include some animal products), and Dietary Approaches to Stop Hypertension (DASH) eating patterns are associated with a lower risk of developing type 2 diabetes [xix] , [xx],[xxi],[xxii]. Evidence suggests that the overall quality of food consumed (as measured by the Healthy Eating Index, Alternative Healthy Eating Index, and DASH score), with an emphasis on whole grains, legumes, nuts, fruits, and vegetables and minimal refined and processed foods, is also associated with a lower risk of type 2 diabetes 21,[xxiii] , [xxiv] , [xxv] . Individualized medical nutrition therapy is effective in lowering A1C in individuals diagnosed with prediabetes [xxvi] .
Recommendation
c- Pharmacologic Interventions: Prescribe metformin for prevention of T2DM in adult individuals with prediabetes, 25-59 years, those with high BMI ≥35 kg/m2, higher fasting plasma glucose 100 – 125 mg/dL, higher A1C 5.7 – 6.4%, women with prior GDM. (strong recommendation, high certainty evidence )
Remarks
No pharmacologic agent has been approved by the U.S. Food and Drug Administration for a specific indication of type 2 diabetes prevention. The risk versus benefit of each medication in support of person-centered goals must be weighed in addition to cost, side effects, and efficacy considerations. Metformin has the longest history of safety data as a pharmacologic therapy for diabetes prevention[xxvii] .
Summary of evidence
weight loss through behavior changes in diet and physical activity alone can be difficult to maintain long term6, people at high risk of diabetes may benefit from support and additional pharmacotherapeutics options Metformin was overall less effective than lifestyle modification in the DPP, though group differences declined over time in the DPPOS7, and metformin may be cost-saving over 10 years[xxviii] . In the DPP, metformin was as effective as lifestyle modification in participants with BMI ≥35 kg/m2 and in younger participants aged 25–44 years1. In individuals with a history of GDM in the DPP, metformin and intensive lifestyle modification led to an equivalent 50% reduction in diabetes risk[xxix] . Both interventions remained highly effective during a 10-year follow-up period [xxx] .
By the time of the 15-year follow up (DPPOS), exploratory analyses demonstrated that participants with a higher baseline fasting glucose (≥110 mg/dL vs. 95–109 mg/dL), those with a higher A1C (6.0–6.4% vs. <6.0%), and individuals with a history of GDM (vs. individuals without a history of GDM) experienced higher risk reductions with metformin, identifying subgroups of participants that benefitted the most from metformin[xxxi] . In the Indian Diabetes Prevention Program (IDPP-1), metformin and lifestyle intervention reduced diabetes risk similarly at 30 months; of note, the lifestyle intervention in IDPP-1was less intensive than that in the DPP [xxxii] . Based on findings from the DPP, metformin should be recommended as an option for high-risk individuals (e.g., those with a history of GDM or those with BMI ≥35 kg/m2). Consider periodic monitoring of vitamin B12 levels in those taking metformin chronically to check for possible deficiency [xxxiii] ,[xxxiv].
Recommendation
d- Prediabetes is associated with heightened cardiovascular risk; therefore, screening for and treatment of modifiable risk factors for cardiovascular disease are suggested. (Strong recommendation, moderate certainty evidence )
Remarks
In people with a history of stroke and evidence of insulin resistance and prediabetes, pioglitazone may be considered to lower the risk of stroke or myocardial infarction. However, this benefit needs to be balanced with the increased risk of weight gain, edema, and fracture. A lower dose may mitigate the risk of adverse effects.
Summary of evidence
People with prediabetes often have other cardiovascular risk factors, including hypertension and dyslipidemia[xxxv] , and are at increased risk for cardiovascular disease [xxxvi] , [xxxvii] . If indicated, evaluation for tobacco use and referral for tobacco cessation should be part of routine care for those at risk for diabetes.
In longer-term follow-up, lifestyle interventions for diabetes prevention also prevented the development of microvascular complications among women enrolled in the DPPOS and in the study population enrolled in the China Da Qing Diabetes Prevention Outcome Study7,[xxxviii] .
The lifestyle intervention in the latter study was also efficacious in preventing cardiovascular disease and mortality at 23 and 30 years of follow-up3,5. Treatment goals and therapies for hypertension and dyslipidemia in the primary prevention of cardiovascular disease for people with prediabetes should be based on their level of cardiovascular risk. Increased vigilance is warranted to identify and treat these and other cardiovascular disease risk factors[xxxix] . Statins have been associated with a modestly increased risk of diabetes [xl] , [xli], [xlii], [xliii], [xliv]. In the DPP, statin use was associated with greater diabetes risk irrespective of the treatment group (pooled hazard ratio [95% CI] for incident diabetes 1.36 [1.17–1.58]) 42. In studies of primary prevention of cardiovascular disease, cardiovascular and mortality benefits of statin therapy exceed the risk of diabetes[xlv] , [xlvi]
In studies of primary prevention of cardiovascular disease, cardiovascular and mortality benefits of statin therapy exceed the risk of diabetes45,46, suggesting a favorable benefit to harm balance with statin therapy.
Recommendation
e- More intensive preventive approaches should be considered in individuals who are at particularly high risk of progression to diabetes, including individuals with BMI ≥35 kg/m2, those at higher glucose levels (e.g., fasting plasma glucose 100–125 mg/dL, 2-h postprandial glucose 140– 199 mg/dL, A1C 5.7 -6.4%), and individuals with a history of gestational diabetes mellitus. (Strongly recommendation, high certainty evidence )
f- Pharmacotherapy should be considered to achieve sustained weight loss, minimize the progression of hyperglycemia, and cardiovascular risk reduction. (strong recommendation, moderate certainty evidence)
Remark
It is important to individualize the risk/benefit of intervention and consider person-centered goals. Risk models have explored risk-based benefit, generally finding the higher benefit of the intervention in those at highest risk9.
Summary of evidence
Individualized risk/benefit should be considered in screening, intervention, and monitoring to prevent or delay type 2 diabetes and associated comorbidities. Multiple factors, including age, BMI, and other comorbidities, may influence the risk of progression to diabetes and the lifetime risk of complications[xlvii] ,[xlviii]. In the DPP, which enrolled high-risk individuals with impaired glucose tolerance, elevated fasting glucose, and elevated BMI, the crude incidence of diabetes within the placebo arm was 11.0 cases per 100 person-years, with a cumulative 3-year incidence of diabetes of 28.9%1 . Characteristics of individuals in the DPP/ DPPOS who were at particularly high risk of progression to diabetes (crude incidence of diabetes 14–22 cases/100 person years) included BMI ≥35 kg/m2, those at higher glucose levels (e.g., fasting plasma glucose 110–125 mg/dL, 2-h postchallenge glucose 173–199 mg/dL, and A1C ≥6.0%), and individuals with a history of gestational diabetes1,29, 30. In contrast, in the community-based Atherosclerosis Risk in Communities (ARIC) study, observational follow-up of older adults (mean age 75 years) with laboratory evidence of prediabetes (based on A1C 5.7–6.4%and/or fasting glucose 100–125 mg/dL), but not meeting specific BMI criteria, found much lower progression to diabetes over 6 years: 9% of those with A1C defined prediabetes, 8% with impaired fasting glucose48.
[i] Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl JMed 2002;346:393–403.
[ii] Lindstr€om J, Ilanne-Parikka P, Peltonen M, et al.; Finnish Diabetes Prevention Study Group.Sustained reduction in the incidence of type 2diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006; 368: 1673–1679
[iii] Li G, Zhang P, Wang J, et al. Cardiovascularmortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–480
[iv] Nathan DM, Bennett PH, Crandall JP, et al.;DPP Research Group. Does diabetes prevention translate into reduced long-term vascular complications of diabetes? Diabetologia 2019;62: 1319–1328
[v] Gong Q, Zhang P, Wang J, et al.; Da Qing Diabetes Prevention Study Group. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results ofthe Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol 2019;7:452–461
[vi] Knowler WC, Fowler SE, Hamman RF, et al.; Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686
[vii] Diabetes Prevention Program Research Group;Nathan DM, Barrett-Connor E, Crandall JP, et al. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications: the DPP Outcomes Study. Lancet Diabetes Endocrinol 2015;3:866–875
[viii] Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP):description of lifestyle intervention. Diabetes Care 2002;25:2165–2171
[ix] Hamman RF,Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102–2107
[x] Ratner RE, Christophi CA, Metzger BE, et al.; Diabetes Prevention Program Research Group. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008;93:4774–4779
[xi] Aroda VR, Christophi CA, Edelstein SL, et al.;Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab 2015;100:1646–1653
[xii] Diabetes Prevention Program Research Group. Long-term effects of metformin on diabetes prevention: identification of subgroups that benefited most in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care 2019;42:601–608
[xiii] Thorp AA, Kingwell BA, Sethi P, Hammond L, Owen N, Dunstan DW. Alternating bouts of sitting and standing attenuate postprandial glucose responses. Med Sci Sports Exerc 2014;46:2053–2061
[xiv] Healy GN, Dunstan DW, Salmon J, et al.Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care 2008;31:661–666
[xv] Russo LM, Nobles C, Ertel KA, Chasan-Taber L,Whitcomb BW. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: a systematic review and meta-analysis. Obstet Gynecol 2015;125:576–582
[xvi] Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019;42:731–754
[xvii] Department of Health and Human Services and Department of Agriculture. Dietary Guidelines for Americans 2015–2020, Eighth Edition. Accessed 12 October 2022. Available from https://www.health.gov/dietaryguidelines/2015/guidelines
[xviii] Salas-Salvado J, Guasch-Ferre M, Lee C-H, Estruch R, Clish CB, Ros E. Protective effects of the Mediterranean diet on type 2 diabetes and metabolic syndrome. J Nutr 2016;146:920S–927S
[xix] Bloomfield HE, Koeller E, Greer N,MacDonald R, Kane R,Wilt TJ. Effects on health outcomes of a Mediterranean diet with no restriction on fat intake: a systematic review and meta-analysis. Ann Intern Med 2016;165:491–500
[xx] Estruch R, Ros E, Salas-Salvado J, et al.; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018;378:e34
[xxi] Stentz FB, Brewer A,Wan J, et al. Remission of pre-diabetes to normal glucose tolerance in obese adults with high protein versus high carbohydrate diet: randomized control trial. BMJ Open Diabetes Res Care 2016;4:e000258
[xxii] Chiu THT, Pan W-H, Lin M-N, Lin C-L. Vegetarian diet, change in dietary patterns, and diabetes risk: a prospective study. Nutr Diabetes 2018;8:12
[xxiii] Lee Y, Park K. Adherence to a vegetarian diet and diabetes risk: of observational studies. Nutrients 2017;9:E603
[xxiv] Qian F, Liu G, Hu FB, Bhupathiraju SN, Sun Q. Association between plant-based dietary patterns and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA Intern Med 2019;179:1335–1344
[xxv] Esposito K, Chiodini P, Maiorino MI, Bellastella G, Panagiotakos D, Giugliano D.Which diet for prevention of type 2 diabetes? A metaanalysis of prospective studies. Endocrine 2014;47:107–116
[xxvi] Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 2014;383:1999–2007
[xxvii] Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012;35:731–737
[xxviii] Aroda VR, Edelstein SL, Goldberg RB, et al.;Diabetes Prevention Program Research Group. Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 2016; 101:1754–1761
[xxix] Ratner RE, Christophi CA, Metzger BE, et al.; Diabetes Prevention Program Research Group. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008;93:4774–4779
[xxx] Aroda VR, Christophi CA, Edelstein SL, et al.; Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab 2015;100:1646–1653
[xxxi] Diabetes Prevention Program Research Group. Long-term effects of metformin on diabetes prevention: identification of subgroups that benefited most in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care 2019;42:601–6
[xxxii] Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD; Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297
[xxxiii] Griffin SJ, Bethel MA, Holman RR, et al. Metformin in non-diabetic hyperglycaemia: the GLINT feasibility RCT. Health Technol Assess 2018;22:1–64.
[xxxiv] Aroda VR, Edelstein SL, Goldberg RB, et al.; Diabetes Prevention Program Research Group. Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 2016; 101:1754–1761.
[xxxv] Ali MK, Bullard KM, Saydah S, Imperatore G, Gregg EW. Cardiovascular and renal burdens of prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988-2014. Lancet Diabetes Endocrinol 2018;6:392–403.
[xxxvi] Pan Y, Chen W, Wang Y. Prediabetes and outcome of ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis 2019;28:683–692
[xxxvii] Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ 2016; 355:i5953
[xxxviii] Gong Q, Gregg EW, Wang J, et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia 2011;54:300–307.
[xxxix] Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primaryprevention of cardiovascular disease: a report ofthe American College of Cardiology/ American Heart Association Task Force on Clinical PracticeGuidelines. Circulation 2019;140:e596–e646.
[xl] Thakker D, Nair S, Pagada A, Jamdade V, Malik A. Statin use and the risk of developing diabetes: a network meta-analysis. Pharmacoepidemiol Drug Saf 2016;25:1131–1149
[xli] Macedo AF, Douglas I, Smeeth L, Forbes H, Ebrahim S. Statins and the risk of type 2 diabetes mellitus: cohort study using the UK Clinical Practice Research Datalink. BMC Cardiovasc Disord 2014; 14:85
[xlii] Crandall JP, Mather K, Rajpathak SN, et al. Statin use and risk of developing diabetes: results from the Diabetes Prevention Program. BMJ Open Diabetes Res Care 2017;5:e000438
[xliii] Preiss D, Seshasai SRK, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011;305:2556–2564
[xliv] Mansi IA, Chansard M, Lingvay I, Zhang S, Halm EA, Alvarez CA. Association of statin therapy initiation with diabetes progression: a retrospective matched-cohort study. JAMA Intern Med 2021;181:1562–1574
[xlv] Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012;380:565–571
[xlvi] Cai T, Abel L, Langford O, et al. Associations between statins and adverse events in primary prevention of cardiovascular disease: systematic review with pairwise, network, and doseresponse meta-analyses. BMJ 2021;374:n1537
[xlvii] Nadeau KJ, Anderson BJ, Berg EG, et al. Youth-onset type 2 diabetes consensus report: current status, challenges, and priorities. Diabetes Care 2016;39:1635–1642
[xlviii] Rooney MR, Rawlings AM, Pankow JS, et al. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med 2021; 181:511–519
Appendix 1
Further readings
- Mediterranean diet and diabetes https://patientinfo.org.au
- Rational for the use of a Mediterranean diet in diabetes management https://diabetesjournals.org
- Nutritional recommendation for individuals with diabetes https://www.ncbi.nlm.nih.gov
-DASH eating plan: An eating pattern for diabetes management diabetes journals.org
- Implementation considerations
Several barriers may hinder the effective implementation and scale-up of the recommendations in this guideline. These factors may be related to the behaviours of patients (or families), the behavior of healthcare professionals, the organization of care, health service delivery or financial arrangements.
Obstacles to effective implementation include:
▪️ Patient engagement
▪️ Collaboration; person centered, team based collaboration between clinician, dietitian, pharmacist and others involved in care delivery
▪️ Behavior changes: information, guidance and support delivered easily and consistently can help assess sustained behavioral changes.
▪️ Increase Awareness through educational programs development of prediabetes educational program is recommended and should be encouraged by different healthcare sectors which may include, but not limited to, awareness days, social campaigns, and printed materials.
➡️Research needs
During the review of evidence and the development of recommendations, several research gaps were identified regarding the Egyptian population considered as a limitation of the body of evidence. Addressing these will help inform the revision of these guidelines.
1. Tailoring Interventions: Investigate the effectiveness of personalized or tailored interventions within diabetes prevention programs. Explore how individual characteristics, cultural factors, socioeconomic status, and health literacy influence program outcomes and identify strategies for optimizing intervention customization.
2. Technology-Based Approaches: Evaluate the effectiveness of technology-based interventions, such as mobile applications, wearable devices, and telehealth platforms, in enhancing diabetes prevention efforts. Assess the feasibility, acceptability, and impact of these technologies on participant engagement, behavior change, and long-term outcomes.
3. Adherence and Retention: Investigate strategies to improve participant adherence and retention in diabetes prevention programs. Understand the factors influencing program attrition and develop interventions to enhance engagement, motivation, and long-term commitment.
4. Comparative Effectiveness: Conduct comparative effectiveness research to compare different types of diabetes prevention programs, such as group-based interventions, individual counseling, online programs, or community-based initiatives. Evaluate their relative efficacy, cost-effectiveness, and suitability for different populations.
- Monitoring and evaluating the impact of the guideline
There are potential indicators that can be used to monitor and evaluate the success of implementing a diabetes prevention program through the Egyptian Health Council (EHC). These indicators can provide complementary information and a more comprehensive assessment of the program's effectiveness.
1. Changes in Body Mass Index (BMI): Track changes in average BMI or the proportion of individuals classified as overweight or obese within the target population. Obesity is a significant risk factor for developing type 2 diabetes, and a reduction in BMI indicates improvements in weight management and overall health.
2. Blood Glucose Control: Measure average blood glucose levels or the proportion of individuals with well-controlled blood glucose within the target population. Improved blood glucose control indicates better management of diabetes risk factors and reduced progression to diabetes.
3. Program Engagement and Participation: Evaluate the level of engagement and participation in the prevention program, including attendance rates for educational sessions, participation in physical activity programs, and utilization of support resources. Higher engagement indicates increased program reach and potential effectiveness.
➡️Updating of the guidelines:
These guidelines will be updated whenever there is new evidence.
- Reference
Adapted from ADA “Standards of Care in Diabetes” 2024
[1] Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl JMed 2002;346:393–403.
[1] Lindstr€om J, Ilanne-Parikka P, Peltonen M, et al.; Finnish Diabetes Prevention Study Group.Sustained reduction in the incidence of type 2diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006; 368: 1673–1679
[1] Li G, Zhang P, Wang J, et al. Cardiovascularmortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–480
[1] Nathan DM, Bennett PH, Crandall JP, et al.;DPP Research Group. Does diabetes prevention translate into reduced long-term vascular complications of diabetes? Diabetologia 2019;62: 1319–1328
[1] Gong Q, Zhang P, Wang J, et al.; Da Qing Diabetes Prevention Study Group. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results ofthe Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol 2019;7:452–461
[1] Knowler WC, Fowler SE, Hamman RF, et al.; Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686
[1] Diabetes Prevention Program Research Group;Nathan DM, Barrett-Connor E, Crandall JP, et al. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications: the DPP Outcomes Study. Lancet Diabetes Endocrinol 2015;3:866–875
[1] Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP):description of lifestyle intervention. Diabetes Care 2002;25:2165–2171
[1] Hamman RF,Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102–2107
[1] Ratner RE, Christophi CA, Metzger BE, et al.; Diabetes Prevention Program Research Group. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008;93:4774–4779
[1] Aroda VR, Christophi CA, Edelstein SL, et al.;Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab 2015;100:1646–1653
[1] Diabetes Prevention Program Research Group. Long-term effects of metformin on diabetes prevention: identification of subgroups that benefited most in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care 2019;42:601–608
[1] Thorp AA, Kingwell BA, Sethi P, Hammond L, Owen N, Dunstan DW. Alternating bouts of sitting and standing attenuate postprandial glucose responses. Med Sci Sports Exerc 2014;46:2053–2061
[1] Healy GN, Dunstan DW, Salmon J, et al.Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care 2008;31:661–666
[1] Russo LM, Nobles C, Ertel KA, Chasan-Taber L,Whitcomb BW. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: a systematic review and meta-analysis. Obstet Gynecol 2015;125:576–582
[1] Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019;42:731–754
[1] Department of Health and Human Services and Department of Agriculture. Dietary Guidelines for Americans 2015–2020, Eighth Edition. Accessed 12 October 2022. Available from https://www.health.gov/dietaryguidelines/2015/guidelines
[1] Salas-Salvado J, Guasch-Ferre M, Lee C-H, Estruch R, Clish CB, Ros E. Protective effects of the Mediterranean diet on type 2 diabetes and metabolic syndrome. J Nutr 2016;146:920S–927S
[1] Bloomfield HE, Koeller E, Greer N,MacDonald R, Kane R,Wilt TJ. Effects on health outcomes of a Mediterranean diet with no restriction on fat intake: a systematic review and meta-analysis. Ann Intern Med 2016;165:491–500
[1] Estruch R, Ros E, Salas-Salvado J, et al.; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018;378:e34
[1] Stentz FB, Brewer A,Wan J, et al. Remission of pre-diabetes to normal glucose tolerance in obese adults with high protein versus high carbohydrate diet: randomized control trial. BMJ Open Diabetes Res Care 2016;4:e000258
[1] Chiu THT, Pan W-H, Lin M-N, Lin C-L. Vegetarian diet, change in dietary patterns, and diabetes risk: a prospective study. Nutr Diabetes 2018;8:12
[1] Lee Y, Park K. Adherence to a vegetarian diet and diabetes risk: of observational studies. Nutrients 2017;9:E603
[1] Qian F, Liu G, Hu FB, Bhupathiraju SN, Sun Q. Association between plant-based dietary patterns and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA Intern Med 2019;179:1335–1344
[1] Esposito K, Chiodini P, Maiorino MI, Bellastella G, Panagiotakos D, Giugliano D.Which diet for prevention of type 2 diabetes? A metaanalysis of prospective studies. Endocrine 2014;47:107–116
[1] Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 2014;383:1999–2007
[1] Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012;35:731–737
[1] Aroda VR, Edelstein SL, Goldberg RB, et al.;Diabetes Prevention Program Research Group. Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 2016; 101:1754–1761
[1] Ratner RE, Christophi CA, Metzger BE, et al.; Diabetes Prevention Program Research Group. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008;93:4774–4779
[1] Aroda VR, Christophi CA, Edelstein SL, et al.; Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab 2015;100:1646–1653
[1] Diabetes Prevention Program Research Group. Long-term effects of metformin on diabetes prevention: identification of subgroups that benefited most in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care 2019;42:601–6
[1] Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD; Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297
[1] Griffin SJ, Bethel MA, Holman RR, et al. Metformin in non-diabetic hyperglycaemia: the GLINT feasibility RCT. Health Technol Assess 2018;22:1–64.
[1] Aroda VR, Edelstein SL, Goldberg RB, et al.; Diabetes Prevention Program Research Group. Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 2016; 101:1754–1761.
[1] Ali MK, Bullard KM, Saydah S, Imperatore G, Gregg EW. Cardiovascular and renal burdens of prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988-2014. Lancet Diabetes Endocrinol 2018;6:392–403.
[1] Pan Y, Chen W, Wang Y. Prediabetes and outcome of ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis 2019;28:683–692
[1] Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ 2016; 355:i5953
[1] Gong Q, Gregg EW, Wang J, et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia 2011;54:300–307.
[1] Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primaryprevention of cardiovascular disease: a report ofthe American College of Cardiology/ American Heart Association Task Force on Clinical PracticeGuidelines. Circulation 2019;140:e596–e646.
[1] Thakker D, Nair S, Pagada A, Jamdade V, Malik A. Statin use and the risk of developing diabetes: a network meta-analysis. Pharmacoepidemiol Drug Saf 2016;25:1131–1149
[1] Macedo AF, Douglas I, Smeeth L, Forbes H, Ebrahim S. Statins and the risk of type 2 diabetes mellitus: cohort study using the UK Clinical Practice Research Datalink. BMC Cardiovasc Disord 2014; 14:85
[1] Crandall JP, Mather K, Rajpathak SN, et al. Statin use and risk of developing diabetes: results from the Diabetes Prevention Program. BMJ Open Diabetes Res Care 2017;5:e000438
[1] Preiss D, Seshasai SRK, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011;305:2556–2564
[1] Mansi IA, Chansard M, Lingvay I, Zhang S, Halm EA, Alvarez CA. Association of statin therapy initiation with diabetes progression: a retrospective matched-cohort study. JAMA Intern Med 2021;181:1562–1574
[1] Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012;380:565–571
[1] Cai T, Abel L, Langford O, et al. Associations between statins and adverse events in primary prevention of cardiovascular disease: systematic review with pairwise, network, and doseresponse meta-analyses. BMJ 2021;374:n1537
[1] Nadeau KJ, Anderson BJ, Berg EG, et al. Youth-onset type 2 diabetes consensus report: current status, challenges, and priorities. Diabetes Care 2016;39:1635–1642
[1] Rooney MR, Rawlings AM, Pankow JS, et al. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med 2021; 181:511–519
- Appendix 1
Further readings
-Mediterranean diet and diabetes https://patientinfo.org.au
--Rational for the use of a Mediterranean diet in diabetes management https://diabetesjournals.org
-Nutritional recommendation for individuals with diabetes https://www.ncbi.nlm.nih.gov
-DASH eating plan: An eating pattern for diabetes management diabetes journals.org