Hepatocellular Carcinoma (HCC)
| Site: | EHC | Egyptian Health Council |
| Course: | Oncology and Hematological Oncology Guidelines |
| Book: | Hepatocellular Carcinoma (HCC) |
| Printed by: | Guest user |
| Date: | Monday, 23 December 2024, 9:23 PM |
Description
"last update: 28 April 2024"
- Committee
• Chair of the Oncology Committee of Egyptian health council Guidelines: Prof Hussein Khaled.
• The Oncology Committee Members: Emad Hamada, Samir Shehata, Hesham Elghazaly, Hesham Tawfik, Fouad Abuotaleb, Ebtesam Saad Eldin, Ihab Khalil, Khaled Abdelkarim, Lobna ezz Elarab, Mary Gamal, Mohamed Abdel Mooti, Mohamed Gamil, Nervana Hussein, Ola Khorshid, Omar Sherif Omar, Rasha Fahmi, Rasha Shaltout, Yousri Wasef & Yousri Rostom.
• Chair of the HCC Scientific Committee: Yousri Rostom
• The HCC Scientific Group Members: Amr Abdel Moety, Ashraf Omar, Gamal Esmat, Hussein Khaled, Fouad Aboutaleb, Khaled Abdelkarim, Mahmoud Elmetainy, Mary Gamal, Mohamed Shaker, Osama Elmalt, & Yousri Wasef.
➡️ Abbreviations
AFP (alphafetoprotein)
ALT(alanine aminotransferase)
AST(aspartate aminotransferase)
CBC (complete blood picture) BCLC (Barcelona Clinic Liver Cancer) cHCC-CCA (combined hepatocellularcarcinoma-cholangiocarcinoma).
CT (computed tomography)
ECOG (eastern cooperative oncology group)
FDG-PET Scan (fluorodeoxyglucose -positron emission tomography scan)
HbA1c(hemoglobin A1c or glycosylated hemoglobin)
HBV (hepatitis B virus)
HCC (hepatocellular carcinoma) HCV (hepatitis virus)
iCCA (intrahepatic cholangiocarcinoma)
INR (international normalized ratio) MDT (multi disciplinary team)
mRECIST(modified response evaluation criteria in solid tumors
MRI (magnetic resonance imaging)
MWA (microwave ablation)
NAFLD (non alcoholic fatty liver disease)
PET (positron emission tomography)
PT (prothrombin time)
PS (performance status)
RECIST (response evaluation criteria in solid tumors)
RFA (radiofrequency ablation)
RT (radiotherapy)
SBRT (stereotactic body radiotherapy)
SRS (stereotactic radio surgery)
UNOS (united network for organ sharing)
TACE (trans arterial chemoemblisation)
TARE (transarterial radio embolization)
- Executive Summary
This guidance provides a data-supported approach to the primary prevention screening, diagnosis, staging, treatment and follow up of patients diagnosed with hepatocellular carcinoma (HCC).
|
Recommendations |
Strength of recommendations |
|
Vaccination against hepatitis B reduces the risk of HCC and is recommended for all newborns and high-risk groups . |
Strong |
|
Governmental health agencies should implement policies to prevent HCV/HBV transmission, counteract chronic alcohol abuse, and encourage life styles that prevent obesity and metabolic syndrome. |
Strong |
|
In general, chronic liver disease should be treated to avoid progression of liver disease. |
Strong |
|
In patients with chronic hepatitis, antiviral therapies leading to maintained HBV suppression in chronic hepatitis B and sustained viral response in hepatitis C are recommended, since they have been shown to prevent progression to cirrhosis and HCC development. |
Strong |
|
Once cirrhosis is established, antiviral therapy is beneficial in preventing cirrhosis progression and decompensation. Furthermore, successful antiviral therapy reduces but does not eliminate the risk of HCC development . |
Strong |
|
Patients with HCV-associated cirrhosis and HCC treated with curative intent, maintain a high rate of HCC recurrence even after subsequent DAA therapy resulting in sustained viral response. close surveillance is advised in these patients. |
Strong |
|
Coffee consumption has been shown to decrease the risk of HCC in patients with chronic liver disease. In these patients, coffee consumption should be encouraged. |
Strong |
|
Implementation of screening programs to identify at- risk candidate populations should be improved. Such programs are a public health goal, aiming to decrease HCC-related and overall liver-related deaths. |
Strong |
|
Screening for HCC is warranted in all patients with cirrhosis irrespective of aetiology as long as liver function and co-morbidities allow curative or palliative treatment. |
Strong |
|
Screening for HCC is warranted for all patients with chronic HBV, regardless of the fibrosis stage. |
Strong |
|
Screening for HCC is warranted in all patients with advanced fibrosis (F3 or F4) with HCV or NAFLD . |
Strong |
|
Screening of patients at risk of HCC should be carried out by abdominal Us with AFP every 4 months. |
Good practice statement |
|
Patients with liver nodule(s) < 1cm or 1-2 cm [LI-RADS 1or 2]on abdominal ultrasound should repeat short-interval ultrasound and AFP after 3 months. |
Strong |
|
In at-risk patients with any suspicious lesion ≥ 1 cm on ultrasound should undergo diagnostic evaluation with multi-phasic contrast- enhanced CT or contrast-enhanced multi-phasic MRI. |
Strong |
|
All patients with HCC should be carefully discussed and managed by an experienced multidisciplinary team(MDT) with the involvement of hepatologists, diagnostic radiologists, interventional radiologists, surgeons, transplant surgeons ,medical oncologists ,radiation oncologists, pathologists with hepatobiliary cancer expertise ,clinical pharmacists ,nutritionists and palliative care specialists. |
Strong |
|
The noninvasive diagnosis of HCC should be based on either multi-phasic contrast- enhanced CT or dynamic contrast enhanced MRI for diagnosis and evaluation of tumor extent(number and size of nodules,vascular invasion,extra-hepatic spread),they should could be performed,interpreted, and reported through the CT/MRI Liver Imaging Reporting and Data System(CT/MRI LI-RADS). |
Strong |
|
The diagnosis of HCC can be established if the typical vascular hallmarks of HCC (hypervascularity in the arterial phase with washout in the portal venous or delayed phase) are identified in a nodule of >1 cm diameter using one of the two contrast enhancing imaging techniques, either CT or MRI, in a cirrhotic patient. |
strong |
|
The optimal diagnostic method is core biopsy.Indicators for consideration of core needle biopsy include: • lesion> 1cm in cirrhotic patients but does not meet imaging criteria for HCC in multi-phasic CT and MRI. • lesion meets imaging criteria for HCC but patients is not considered at high risk for HCC development(In non-cirrhotic patients). • lesion meets imaging criteria for HCC but patient has elevated CA19-9 or CEA with suspicion of iCCA or cHCC-CCA. |
Conditional |
|
Repeated bioptic sampling is recommended in cases of inconclusive histological or discordant findings, or in cases of growth or change in enhancement pattern identified during follow-up, but with imaging still not diagnostic for HCC. |
Conditional |
|
Staging of HCC is important to determine outcome and planning of optimal therapy and includes assessment of tumor extent,AFP, liver function,portal pressure and clinical performance status. |
strong |
|
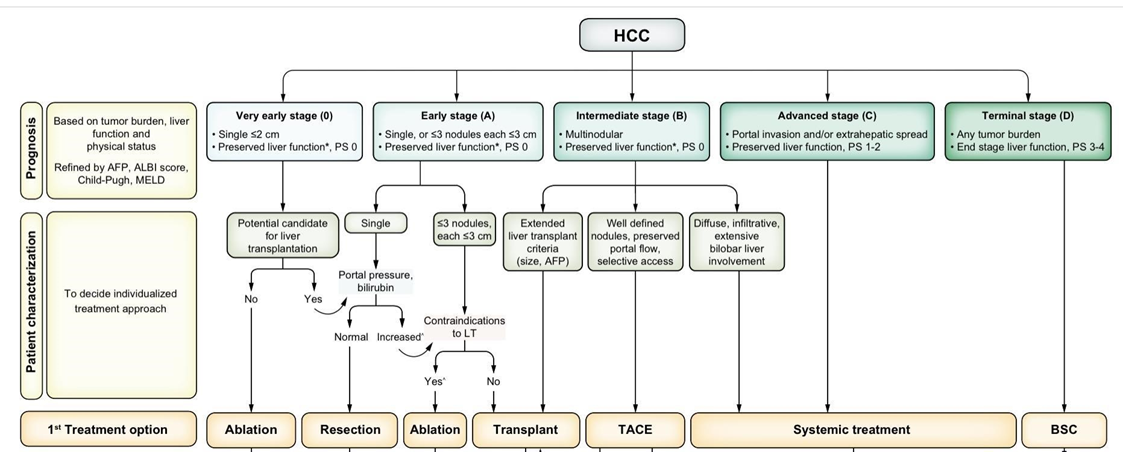
The Barcelona Clinic Liver Cancer (BCLC) is the commonly accepted staging system for prognostic prediction and treatment allocation. |
strong |
|
Multi-phasic contrast-enhanced CT or MRI of the abdomen, CT of the chest, and CT/MRI of the pelvis are also used in the evaluation of the HCC tumor burden to detect the presence of metastatic disease. |
Conditional |
|
Initial workup for patients with suspected HCC is a multidisciplinary evaluation including careful review of medical history to identify any potential chronic liver diseases, investigations of the etiologic origin of liver disease, an assessment of the presence of comorbidity, imaging studies to detect the presence of metastatic disease, and an evaluation of hepatic function, including a determination of whether portal hypertension is present. |
Conditional |
|
Laboratory evaluation of patients with newly diagnosed HCC include testing to detect Aetiology of liver disease: HBV (at least HBsAg and anti-HBc), HCV (at least anti-HCV), iron status, autoimmune profile,HbAIc,others as indicated. |
Good practice statement |
|
Initial Workup for patient with HCC include an initial assessment of hepatic function involves liver function testing including measurement of serum levels of bilirubin, AST, ALT, ALP, measurement of PT expressed as INR, albumin, and platelet count (surrogate for portal hypertension). Other recommended tests include CBC, BUN, and creatinine to assess kidney function. |
Strong |
|
Endoscopic assessment of any HCC patient: Upper GIT endoscopy is advised before receiving systemic therapy or surgery. |
Conditional |
|
FDG PET-scan is not recommended for early diagnosis of HCC because of the high rate of false negative cases and may be considered when there is an equivocal extrahepatic finding before liver transplant. |
Strong |
|
Partial hepatectomy should be offered to HCC patients without advanced fibrosis and is the treatment of choice as long as an R0-resection can be carried out. |
Strong |
|
In the case of cirrhosis, surgical treatment is recommended for localized HCC with a single lesion and intact liver function (Child-Pugh A), and in the absence of clinically significant portal hypertension with the evaluation of the extent of hepatectomy,future liver revenant and patient performance status. |
strong |
|
For patients with chronic liver disease being considered for major resection, preoperative portal vein embolization should be considered. |
strong |
|
Patients meeting the UNOS criteria [AFP level ≤1000 ng/mL and single lesion ≥2 cm and ≤5 cm, or 2 or 3 lesions ≥1 cm and ≤3 cm and no evidence of macro vascular involvement or extra-hepatic disease] should be considered for liver transplantation. |
strong |
|
Thermal ablation by RFA or MWA are recommended as an alternative for resection for a single nodule ≤ 3 cm, BCLC stage 0, and those early stages that are not candidates for resection. |
Strong |
|
The number and diameter of lesions treated by RFA in one session should not exceed three lesions, 3 cm each. |
Conditional |
|
Unresectable lesions measuring up to 4 cm are recommended to be subject to local ablative therapy by radiofrequency ablation (RFA) or microwave ablation. |
Strong |
|
Percutaneous ethanol injection is considered an option in some cases of very early HCC with tumor size up to 2 cm when thermal ablation is not technically feasible. |
Strong |
|
EBRT (i.e. IMRT, SRS/SBRT) is recommended as a potential first line single option for patients with liver-confined HCC who are not candidates for curative options (surgery or thermal ablation) and for whom TACE is being considered. |
Strong |
|
Single lesions (4–6 cm) that are beyond local ablative therapy and are ineligible for surgical resection and transplantation could benefit from a combination of heat ablation and chemoembolization and/or radiotherapy. |
Strong |
|
TACE may be considered as an eligible option in intermediate HCC for bridging and down staging before liver transplantation and in case of non-feasibility or failure of other curative options in single lesions up to 8 cm. |
Conditional |
|
TACE is recommended for BCLC-B patients with Child score up to B7 and tumor burden less than 50 % of liver volume |
Strong |
|
TACE should not be recommended for patients with decompensated liver disease (Child-Pugh score > 7), advanced liver and/or kidney dysfunction, main portal vein or its main branches invasion, extrahepatic spread, or tumor occupying>50 % of the liver size. |
Strong |
|
TACE should not be repeated after two consecutive sessions, with at least one month interval, and there is no response or there is tumor progression or decompensation of liver beyond Child-Pugh score B7. |
Conditional |
|
Transarterial bland embolization may be used in same indications of TACE as A second choice if TACE is not feasible. |
Conditional |
|
Radiotherapy in HCC is recommended to be integrated in the treatment plan through expert MDT and should be carried out in well trained and equipped centers with image guided, stereotactic radiotherapy, and radiosurgery facilities. |
Strong |
|
Radiotherapy could be implemented for unresectable or medically inoperable disease irrespective of the location (3D conformal RT, intensity-modulated RT [IMRT], or stereotactic body RT [SBRT]). |
Strong |
|
To give radiotherapy, there should be no extrahepatic disease or it should be minimal and addressed in a comprehensive management plan. Those with Child-Pugh B (max 7) cirrhosis can be safely treated, but they may require dose modifications and strict dose constraint adherence. |
Strong |
|
Image-guided RT is strongly recommended to improve treatment accuracy and reduce treatment related toxicity. |
Strong |
|
SBRT or SRS can be considered after ablation/ embolization techniques have failed or are contraindicated. |
Strong |
|
SBRT (typically 3–5 fractions) is recommended for patients with 1 to 3 tumors. And could be considered for larger lesions or more extensive disease, if there is sufficient uninvolved liver and liver radiation tolerance can be respected. |
Conditional |
|
SBRT or SRS are recommended for compensated cirrhotic patients with HCC and portal vein thrombosis and when patients are ineligible for other modalities with building-up results. |
Conditional |
|
Palliative RT is indicated for symptomatic control and/or prevention of complications from metastatic lesions as bone or brain, and extensive liver tumor burden. |
Strong |
|
The recommended doses of radiotherapy should be based on meeting normal organ constraints and underlying liver function as follows: ▪️ SBRT, SRS: 30–50 Gy (typically in 3–5 fractions) ▪️ Hypofractionation: 37.5–72 Gy in 10–15 fractions ▪️ Conventional fractionation by IMRT: 50–66 Gy in 25–33 fractions |
Strong |
|
Systemic therapy should be offered to patients with preserved liver function (Child-Turcotte Pugh A or well-selected Child-Turcotte-Pugh B cirrhosis),ECOG PS0-1,who have BCLC Stage C HCC,or BCLC Stage B HCC not amenable to or progressing after locoregional therapy. |
Strong |
|
Sorafenib is the standard of care as first line for patients with advanced HCC and those with intermediate-stage (BCLC B) disease not eligible for, or progressing despite, locoregional therapies. It is recommended in patients with well-preserved liver function and ECOG PS 0-2. |
Strong |
|
Regorafenib is the standard of care for patients with advanced HCC who have tolerated sorafenib but progressed. It is recommended in patients with well- preserved liver function and ECOG PS 0-1. |
Strong |
|
Patients with BCLC-Stage-D HCC should receive the best supportive care (BSC), including pain management, palliative radiotherapy for painful bone metastasis, nutrition optimization, and psychological support |
Conditional |
|
Follow-up of patients who underwent radical treatments should consist of clinical evaluation with, multi-phasic, high-quality, cross-sectional imaging of the chest, abdomen, and pelvis(ie,CT or MRI) every 3 to 6 months for 2 years, then every 6 months and AFP should be measured every 3 to 6 months for 2 years, then every 6 months. Surveillance imaging and AFP should continue for at least 5 years and thereafter screening is dependent on HCC risk factors. |
Conditional |
|
Follow-up of patients with advanced stages of HCC treated with systemic therapies or locoregional treatment , periodic response assessment with cross-sectional imaging including chest, multiphase abdomen, pelvis and serum level of AFP (every 3 months) |
Good practice statement |
|
Using the mRECIST Criteria in the assessment of progression and radiological response after HCC management is recommended. |
Conditional |
- Introduction
HCC is the most common tumour diagnosed in Egypt among males. It is also of the main causes of cancer related deaths. According to GLOBOCAN last census, the number of new cases of liver cancer in both sexes, all ages is about 28,000 new cases in the year 2020 representing 27.3% of male and 10.5% of female cancers.
➡️ Purpose and scope
These guidelines are developed to improve the quality of care for HCC patients Via providing a uniform standard of care across the country to help in primary prevention, screening, early diagnosis for HCC and so less aggressive treatment options and improved clinical outcomes.
These guidelines cover primary prevention, screening ,diagnosis, staging, treatment and follow-up of HCC.
➡️ Target audience
Clinicians who are involved in the care and treatment of
patients with advanced HCC, including medical oncologists, radiation
oncologists, clinical oncologist, hepatologists, gastroenterologists, surgeons,
interventional radiologists, radiologists, pathologists, and palliative care
specialists.
- Methodology
◾ A comprehensive search for guidelines was undertaken to identify the most relevant guidelines to consider for adaptation.
◾ inclusion/exclusion criteria followed in the search and retrieval of guidelines to be adapted:
- Selecting only evidence-based guidelines (guideline must include a report on systematic literature searches and explicit links between individual recommendations and their supporting evidence).
- Selecting only national and/or international guidelines.
- Specific range of dates for publication (using Guidelines published or updated 2015 and later).
- Selecting peer reviewed publications only.
- Selecting guidelines written in English language.
- Excluding guidelines written by a single author not on behalf of an organization in order to be valid and comprehensive, a guideline ideally requires multidisciplinary input.
- Excluding guidelines published without references as the panel needs to know whether a thorough literature review was conducted and whether current evidence was used in the preparation of the recommendations.
◾ All retrieved Guidelines were screened and appraised using AGREE II instrument (www.agreetrust.org) by at least two members. the panel decided a cut-off point or rank the guidelines (any guideline scoring above 50% on the rigour dimension was retained)
- Evidence assessment
According to WHO handbook for Guidelines we used the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach to assess the quality of a body of evidence, develop and report recommendations. GRADE methods are used by WHO because these represent internationally agreed standards for making transparent recommendations. Detailed information on GRADE is available through the on the following sites:
▪️ GRADE working group: http://www.gradeworkingroup.org
▪️ GRADE online training modules: http://cebgrade.mcmaster.ca/
▪️GRADE profile software: http://ims.cochrane.org/revman/gradepro
◾ Table 1: Quality of evidence in GRADE
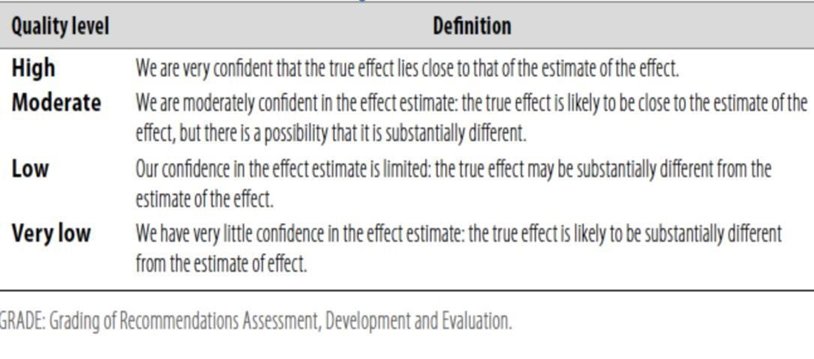
◾ Table 2: Significance of the four levels of evidence
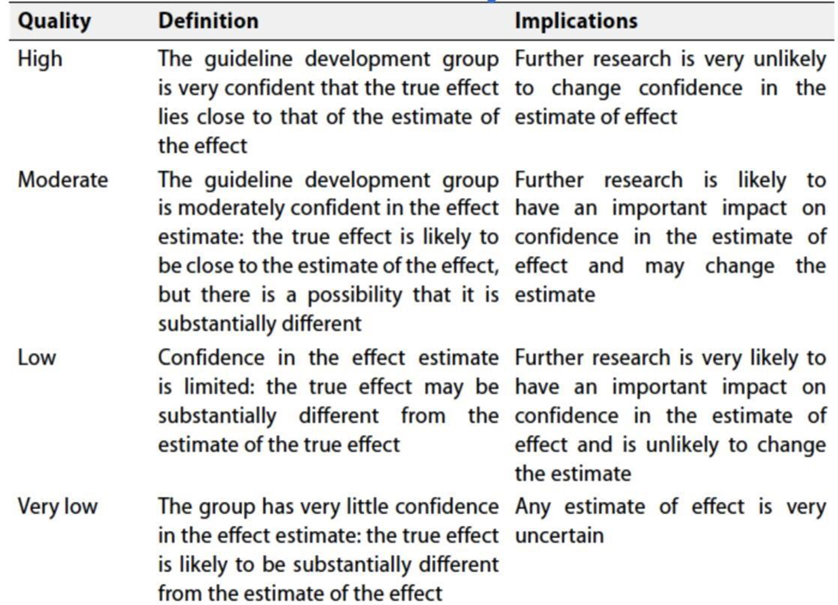
◾ Table 3: Factors that determine How to upgrade or downgrade the quality of evidence

- The strength of the recommendation
The strength of a recommendation communicates the importance of adherence to the recommendation:
➡️ Strong recommendations
With strong recommendations, the guideline communicates the message that the desirable effects of adherence to the recommendation outweigh the undesirable effects. This means that in most situations the recommendation can be adopted as policy.
➡️ Conditional recommendations
These are made when there is greater uncertainty about the four factors above or if local adaptation must account for a greater variety in values and preferences, or when resource use makes the intervention suitable for some, but not for other locations. This means that there is a need for substantial debate and involvement of stakeholders before this recommendation can be adopted as policy.
➡️ When not to make recommendations.
When there is lack of evidence on the effectiveness of an intervention, it may be appropriate not to make a recommendation.
- Recommendations
1. Primary prevention of HCC.
1a. Vaccination against hepatitis B reduces the risk of HCC and is recommended for all new-born and high-risk groups.
Strong recommendation, Moderate Quality Evidence(Observational study), (1)
1b. Governmental health agencies should implement policies to prevent HCV/HBV transmission and encourage lifestyles that prevent obesity and metabolic syndrome.
Strong recommendation, High Quality Evidence(A systematic review ), (2)
1c. In general, chronic liver diseases should be treated to avoid their progression.
Strong recommendation,Moderate Quality Evidence(Narrative review), (3)
1d. In patients with chronic hepatitis, antiviral therapies leading to maintained HBV suppression in chronic hepatitis B and sustained viral response in hepatitis C are recommended, since they have been shown to prevent progression to cirrhosis and HCC development.
Strong recommendation, Moderate Quality Evidence(Narrative review), (3,4)
1e. Once cirrhosis is established, antiviral therapy is beneficial in preventing cirrhosis progression and decompensation. Furthermore, successful antiviral therapy reduces but does not eliminate the risk of HCC development.
Strong recommendation, Moderate Quality Evidence(Observational cohort studies), (5,6)
1f. Patients with HCV-associated cirrhosis and HCC treated with curative intent, maintain a high rate of HCC recurrence even after subsequent DAA therapy resulting in sustained viral response. close surveillance is advised in these patients.
Strong recommendation, Moderate Quality Evidence(observational cohort study), (7)
1g. Coffee consumption has been shown to decrease the risk of HCC in patients with chronic liver disease. In these patients, coffee consumption should be encouraged.
Strong recommendation, Moderate Quality Evidence (Prospective cohort studies), (8,9)
2. Screening of HCC
2a. Implementation of screening programs to identify at-risk candidate populations should be improved. Such programs are a public health goal, aiming to decrease HCC-related and overall liver-related deaths.
Strong recommendation, High Quality Evidence(Randomized controlled Trial), (10)
2b. Screening for HCC is warranted in all patients with cirrhosis irrespective of aetiology as long as liver function and co-morbidities allow curative or palliative treatment .
Strong recommendation, High Quality Evidence(Meta-analysis). (11)
2c. Screening for HCC is warranted for all patients with chronic HBV, regardless of the fibrosis.
Strong recommendation, High Quality Evidence(Systematic review), (12)
2d. Screening for HCC is warranted in all patients with advanced fibrosis (F3 or F4) with HCV or NAFLD.
Strong recommendation, High Quality Evidence( Meat-analysis and Systematic review), (13,14)
2e. Screening of patients at risk of HCC should be carried out by abdominal Us with AFP every 4 months.
Good practice statement
2f. Patients with liver nodule(s) < 1cm or 1-cm [LI- ADS 1or 2] on abdominal ultrasound should repeat short-interval ultrasound and AFP after 3 months.
Strong recommendation, Moderate Quality Evidence(Observational cohort study), (15)
2g. In at-risk patients with any suspicious lesion ≥ 1cm on ultrasound should undergo diagnostic evaluation with multi-phasic contrast- enhanced CT or contrast-enhanced multi-phasic MRI.
Strong recommendation, High Quality Evidence( A systematic review and meta-analysis), (16)
3. Diagnosis and staging of HCC.
3a.
All patients with HCC should be carefully discussed and
managed by an experienced multidisciplinary team(MDT) with the involvement of
hepatologists , diagnostic radiologists, interventional radiologists, surgeons,
transplant surgeons ,medical oncologists ,radiation oncologists, pathologists
with hepatobiliary cancer expertise,clinical pharmacists
nutritionists and palliative care specialists.
Strong recommendation, Moderate Quality Evidence(Observational cohort study), (17)
3b. The
noninvasive diagnosis of HCC should be based on either multiphasic contrast-
enhanced CT or dynamic contrast enhanced MRI for diagnosis and evaluation of tumor
extent(number and size of nodules,vascular invasion,extra-hepatic spread),they should could
be performed,interpreted, and reported through the CT/MRI Liver Imaging Reporting and Data
System(CT/MRI LI-RADS).
Strong recommendation, High Quality Evidence(Meta-analysis), (18)
3c. The diagnosis of HCC can be established if the typical vascular hallmarks of HCC (hypervascularity in the arterial phase with washout in the portal venous or delayed phase) are identified in a nodule of >1 cm diameter using one of the two contrast
enhancing imaging techniques, either CT or MRI, in a cirrhotic patient.
Strong recommendation, High Quality Evidence(Systematic review and meta-analysis), (19)
3d. The optimal diagnostic method is core biopsy.Indicators
for consideration of core needle biopsy include:
• .lesion> 1cm in cirrhotic patients but does not meet imaging criteria for HCC in multi-phasic CT and MRI.
• .lesion meets imaging criteria for HCC but patients is not considered at high risk for HCC development(In non-cirrhotic patients).
• .lesion meets imaging criteria for HCC but patient has elevated CA19-9 or CEA with suspicion of iCCA or cHCC-C
Conditional recommendation, Moderate Quality Evidence(Narrative review), (20)
3e. Repeated bioptic sampling is recommended in cases of inconclusive histological or discordant findings, or in cases of growth or change in enhancement pattern identified during follow-up, but with imaging still not diagnostic for HCC.
Conditional recommendation, Moderate Quality Evidence(Observational cohort study), (21)
3f. Staging of HCC is important to determine outcome and planning of optimal therapy and includes assessment of tumour extent, AFP level, liver function, portal pressure and clinical performance status.
Strong recommendation, High Quality Evidence(Meta-analysis), (22)
3g. The Barcelona Clinic Liver Cancer(BCLC) is the commonly accepted staging system for prognostic prediction and treatment allocation.
Strong recommendation, Moderate Quality Evidence(network meta-analysis of observational studies), (23,24)
3h. Multiphasic contrast-enhanced CT or MRI of the abdomen, CT of the chest, and CT/MRI of the pelvis are also used in the evaluation of the HCC tumor burden to detect the presence of metastatic disease.
Conditional recommendation, Moderate Quality Evidence (Narrative review), (25)
3i.Initial workup for patients with suspected HCC is a multidisciplinary evaluation including careful review of medical history to identify any potential chronic liver diseases, investigations of the etiologic origin of liver disease, an assessment of the presence of comorbidity, imaging studies to detect the presence of metastatic disease, and an evaluation of hepatic function, including a determination of whether portal hypertension is present.
Conditional Recommendation, Moderate Quality Evidence(Meta analysis), (26)
3j.Laboratory evaluation of patients with newly diagnosed HCC include tinvestigations to detect Aetiology of liver disease: HBV (at least HBsAg and anti-HBc), HCV (at least antiHCV), iron status, autoimmune profile, HBA1C, as indicated.
Good practice statement
3k. Initial Workup for patient with HCC include an initial assessment of hepatic function involves liver function testing including measurement of serum levels of bilirubin, AST, ALT, ALP, measurement of PT expressed as INR, albumin, and platelet count (surrogate for portal hypertension). Other recommended tests include CBC, BUN, and creatinine to assess kidney function.
Conditional Recommendation, Moderate Quality Evidence(Observational cohort study), (27)
3l.Endoscopic assessment of any HCC patient: Upper GIT endoscopy is advised before
receiving systemic therapy or surgery.
Conditional Recommendation, Moderate Quality Evidence(observational cohort study), (28)
3m.FDG PET-scan is not recommended for early diagnosis of HCC because of the high rate of false negative cases and may be considered when there is an equivocal extrahepatic finding before liver transplant.
Strong Recommendation, High Quality Evidence(Meta-analysis), (29)
4.1.Management of early HCC
4.1.a. Partial hepatectomy should be offered to HCC patients without advanced fibrosis and is the treatment of choice as long as an R0-resection can be carried out.
Strong recommendation, High Quality Evidence (Meta-analysis), (30)
4.1.b. In the case of cirrhosis, surgical treatment is recommended for localized HCC with a single lesion and intact liver function (Child-Pugh A), and in the absence of clinically significant portal hypertension with the evaluation of the extent of partial hepatectomy, future liver remnant and patient performance status.
Strong recommendation, High Quality Evidence (Meta-analysis and Cohort study), (30,31)
4.1.c For
patients with chronic liver disease being considered for major resection,
preoperative portal vein embolization should be considered.
Strong recommendation, High Quality Evidence(Meta-analysis), (32)
4.1.d. Patients meeting the UNOS criteria ([AFP level ≤1000 ng/mL and single lesion ≥2 cm and ≤5 cm, or 2 or 3 lesions ≥1 cm and ≤3 cm and no evidence of macro vascular involvement or extra-hepatic disease] should be considered for liver transplantation.
Strong recommendation, High Quality Evidence, (33)
4.1.e. Thermal ablation by RFA or MWA are recommended as an alternative for resection for a single nodule ≤ 3 cm, BCLC stage 0, and those early stages that are not candidates for resection.
Strong recommendation, High Quality Evidence(Meta-analysis), (34)
4.1.f. The number and diameter of lesions treated by RFA in one session should not exceed three lesions, 3 cm each.
Conditional recommendation, Moderate Quality Evidence(Narrative review), (35)
4.1.g. Unresectable lesions measuring up to 4 cm are recommended to be subject to local ablative therapy by radiofrequency ablation (RFA) or microwave ablation.
Strong recommendation, HighQuality Evidence (Systematic review), (36)
4.1.h. Percutaneous ethanol injection is considered an option in some cases of very early HCC with tumor size up to 2 cm when thermal ablation is not technically feasible.
Strong recommendation, High Quality Evidence(Systematic review), (37)
4.1.i. EBRT (i.e. IMRT, SRS/SBRT) is recommended as a potential first line single option for patients with liver-confined HCC who are not candidates for curative options (surgery or thermal ablation) and for whom TACE is being considered.
Strong recommendation, Moderate Quality Evidence (systematic review and meta-analysis of
observational studies), (38)
4.1.j. Single lesions (4–6 cm) that are beyond local ablative therapy and are ineligible for surgical resection and transplantation could benefit from a combination of heat ablation and chemoembolization and/or radiotherapy.
Strong recommendation, High Quality Evidence(Metaanalysis of RCTs), (39)
4.1.k. TACE may be considered as an eligible option in intermediate HCC for bridging and down staging before liver transplantation and in case of non-feasibility or failure of other curative options in single lesions up to 8 cm.
Conditional recommendation, Moderate Quality Evidence(Observational cohort study),(40)
4.2.Management of Management of locally advanced/metastatic disease and palliative treatments HCC
4.2.a. TACE is recommended for BCLC-B patients with Child score up to B7 and tumor burden less than 50 % of liver volume.
Strong recommendation, High Quality Evidence(Systematic review), (41)
4.2.b. TACE should not be recommended for patients with decompensated liver disease (Child-Pugh score > 7), advanced liver and/or kidney dysfunction, main portal vein or its main branches invasion, extrahepatic spread, or tumor occupying>50 % of the liver size.
Strong recommendation, High Quality Evidence(Systematic review), (42)
4.2.c. TACE should not be repeated after two consecutive sessions, with at least one month interval, and there is no response or there is tumor progression or decompensation of liver beyond Child-Pugh score B7).
Conditional recommendation, Moderate Quality Evidence(observational cohort study (43)
4.2.d. Transarterial bland embolization may be used in same indications of TACE as A second choice if TACE is not feasible.
Conditional recommendation, Moderate Quality Evidence(observational cohort study),(44)
4.2.e. Radiotherapy in HCC is recommended to be integrated in the treatment plan through expert MDT and should be carried out in well trained and equipped centers with image guided, stereotactic radiotherapy, and radiosurgery facilities.
Strong recommendation, High Quality Evidence(systematic review ),(45)
4.2.f. Radiotherapy could be implemented for unresectable or medically inoperable disease irrespective of the location (3D conformal RT, intensity-modulated RT [IMRT], or stereotactic body RT [SBRT]).
Strong recommendation, High Quality Evidence(systematic review), (45)
4.2.g. To give radiotherapy, there should be no extrahepatic disease or it should be minimal and addressed in a comprehensive management plan. Most of the data on radiation for HCC liver tumors arise from patients with Child-Pugh A liver disease; safety data are limited for patients with Child-Pugh B or poorer liver function. Those with Child-Pugh B (max 7) cirrhosis can be safely treated, but they may require dose modifications and strict dose constraint adherence.
Strong recommendation, Moderate Quality Evidence(observational cohort study), (46)
4.2.h. Image-guided RT is strongly recommended to improve treatment accuracy and reduce treatment related toxicity.
Strong recommendation, Moderate Quality Evidence(observational cohort study), (47)
4.2.i. SBRT or SRS can be considered after ablation/ embolization techniques have failed or are contraindicated.
Strong recommendation, High Quality Evidence(A systematic review and meta analysis), (48)
4.2.j. SBRT (typically 3–5 fractions) is recommended for patients with 1 to 3 tumors. And could be considered for larger lesions or more extensive disease, if there is sufficient uninvolved liver and liver radiation tolerance can be respected.
Conditional recommendation, Moderate Quality Evidence (observational cohort study),(49)
4.2.k. SBRT or SRS are recommended for compensated cirrhotic patients with HCC and portal vein thrombosis and when patients are ineligible for other modalities with building-up results.
Conditional recommendation, Moderate Quality Evidence(observational cohort study), (50)
4.2.l. Palliative RT is indicated for symptomatic control and/or prevention of complications from metastatic lesions as bone or brain, and extensive liver tumor burden.
Strong recommendation, Moderate Quality Evidence(phase 2 trial), (51)
4.2.m. The recommended doses of radiotherapy should be based on meeting normal organ constraints and underlying liver function as follows:
◾ SBRT, SRS: 30–50 Gy (typically in 3–5 fractions)
Strong recommendation,
Moderate Quality Evidence(a systematic review and meta-analysis of
observational studies), (52)
◾ Hypofractionation: 37.5–72 Gy in 10–15 fractions
Strong recommendation,
Moderate Quality Evidence(observational cohort study), (53)
◾ Conventional fractionation by IMRT: 50–66 Gy in 25–33
fractions
Strong recommendation,
High Quality Evidence (a systematic review and metaanalysis), (54)
4.2.n. Systemic therapy should be offered to patients with preserved liver function(ChildTurcotte -Pugh A or well-selected Child-Turcotte-Pugh B cirrhosis),ECOG PS0-1,who have BCLC Stage C HCC,or BCLC Stage B HCC not amenable to or progressing after locoregional therapy.
Strong recommendation, High Quality Evidence(a systematic review and meta-analysis), (55)
4.2.o. Sorafenib is the standard of care as first line for patients with advanced HCC and those with intermediate-stage (BCLC B) disease not eligible for, or progressing despite, locoregional therapies. It is recommended in patients with well-preserved liver function and ECOG PS 0-2.
Strong recommendation, High Quality Evidence(a systematic review ),(56)
4.2.p. Regorafenib is the standard of care for patients with advanced HCC who have tolerated sorafenib but progressed. It is recommended in patients with well- preserved liver function and ECOG PS 0-1.
Strong recommendation, High Quality Evidence(randomized controlled trial)(57)
4.2.q. Patients with BCLC-Stage-D HCC should receive the best supportive care (BSC), including pain management, palliative radiotherapy for painful bone metastasis, nutrition optimization, and psychological support.
Conditional recommendation, low Quality Evidence(review articles) (58,59)
5.Follow up of HCC
5a.Follow-up of patients who underwent radical treatments should consist of clinical evaluation, multiphasic, high-quality, cross-sectional imaging of the chest, abdomen, and pelvis(ie,CT or MRI) every 3 to 6 months for 2 years, then every 6 months and AFP should be measured every 3 to 6 months for 2 years, then every 6 months. Surveillance imaging and AFP should continue for at least 5 years and thereafter screening is dependent on HCC risk factors.
Conditional Recommendation, Moderate Quality Evidence(observational study), (60)
5b. Follow-up of patients with advanced stages of HCC treated with locoregional treatments or systemic therapies, periodic response assessment with cross-sectional imaging including chest, multiphase abdomen, pelvis and serum level of AFP
(every 3 months)
Good practice statement
5c. Using the mRECIST Criteria in the assessment of progression and radiological response after HCC 100% Low management is recommended.
Conditional Recommendation, Moderate Quality Evidence(observational study)(61)
- Clinical indicators for monitoring
▪️ For Screening
1-Abdominal and pelvic ultrasound.
2-Serum alpha -fetoprotein level.
▪️ laboratory evaluation before each cycle of systemic treatment (monthly)
1- complete blood picture.
2-liver function tests (total serum bilirubin, serum albumin, SGPT and INR).
3-kideny function tests (serum creatinine, blood urea)
➡️ Research gaps
• Systematic inclusion of cost-benefit analyses in clinical trial with collection of health economic analysis such as incremental cost effectiveness ratio in order to facilitate clinical decision-making.
• Predictive biomarkers: response to specific systemic targeted therapies.
• Improve models for pre-clinical testing of novel drugs.
• Search for tools to assess quality of life in clinical trials.
• Therapies to prevent dropouts in the waiting lists of liver transplantation and downstaging strategies.
• Adjuvant therapy after curative treatments
➡️Update of this guidline
• This guidline will be updated whenever there is new evidence.
- References
1.Chang M-H,
You S-L, Chen C-J, Liu C-J, Lai M-W, Wu T-C, et al. Long- term effects of hepatitis
B
immunization of infants in preventing liver
cancer. Gastroenterology 2016:21–26.
2. Catharina J Alberts, Gary M
Clifford, Damien Georges, Francesco Negro, Olufunmilayo A Lesi, Yvan J-F Hutin,
Catherine de Martel. Worldwide prevalence of
hepatitis B virus and hepatitis C virus among patients with
Cirrhosis at country, region,and global
levels: a systematic review. Lancet Gastroenterol Hepatol 2022; 7: 724–35.
3. Christoph
Roderburg Frank Tacke Christian Trautwein.Antiviral Therapy in Patients with
Viral Hepatitis and
Hepatocellular Carcinoma: Indications and
Prognosis. Visc Med 2016;32:121–126
2. European Association for the Study of the Liver. EASL
Recommenda- tions on Treatment of Hepatitis C 2018. J Hepatol 2018. https://doi.org/10.1016/j.jhep.2018.03.026.
3. Papatheodoridis GV, Idilman R, Dalekos GN, Buti M, Chi H,
van Boemmel F, et al. The risk of hepatocellular carcinoma decreases after the
first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B.
Hepatology 2017;66:1444–1453.
4.
Su T-H, Hu T-H, Chen C-Y, Huang Y-H,
Chuang W-L, Lin C-C, et al. Four- year entecavir therapy reduces hepatocellular
carcinoma, cirrhotic events and mortality in chronic hepatitis B patients.
Liver Int 2016;36:1755– 1764.
5. van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour J-F,
Lammert F, et al. Association between sustained virological response and
all-cause mortality among patients with chronic hepatitis C and advanced
hepatic fibrosis. JAMA 2012;308:2584.
6. . Inoue M, Yoshimi I, Sobue T, Tsugane S. Influence of
coffee drinking on subsequent risk of hepatocellular carcinoma: a prospective
study in Japan. J Natl Cancer Inst 2005;97:293–300.
7. Setiawan VW, Wilkens LR, Lu SC, Hernandez BY, Le Marchand L,
Henderson BE. Association of coffee intake with reduced incidence of liver
cancer and death from chronic liver disease in the US multiethnic cohort.
Gastroenterology 2015;148:118–25; quiz e15.
8. Zhang B.H., Yang B.H., Tang Z.Y. (2004) Randomized
controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 130: 417– 422.
9. Amit G. Singal, Emily Zhang, Manasa Narasimman, Nicole E.
Rich, Akbar K. Waljee, Yujin Hoshida, Ju Dong Yang, Maria Reig, Giuseppe
Cabibbo, Pierre Nahon, Neehar D Parikh,
Jorge A Marrero, HCC surveillance improves early detection, curative treatment
receipt, and survival in patients with cirrhosis: A metaanalysis,Journal of
Hepatology,Volume 77,Issue1,Julyl
2022,Pages 128-139.
10. Fattovich G, Bortolotti F, Donato F. Natural
history of chronic hepatitis B: special emphasis on disease progression and
prognostic factors. J Hepatol. 2008;48(2):335–52.
11. Nicole J. Kim,1 Philip Vutien, Erin Cleveland,1
Anne Cravero, and George N. Ioannou1. Fibrosis Stage-specific Incidence of
Hepatocellular Cancer After Hepatitis C Cure With Direct-acting Antivirals: A
Systematic Review and Meta-analysis. Clinical Gastroenterology and Hepatology
2023;21:1723–1738.
12. Ioannou GN, Green P, Kerr KF, Berry
K. Models estimating risk of hepatocellular carcinoma in patients with alcohol
or NAFLD- related cirrhosis for risk stratification. J Hepatol 2019;71:523-
533.
15.Singal
AG, Ghaziani T, Zhou K, Grinspan L, Benhammou J, Moon A, et al. Recall patterns
and risk of primary liver cancer for
sub-centimeter ultrasound liverobservations: A multicenter study. Hepatology
Communications.
2023;7(3):e0073.
16. Roberts
LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, et al. Imaging for
the diagnosis of hepatocellular
carcinoma: A systematic review and metaanalysis. Hepatol- ogy. 2018;67:401–21.
17. Dong Hyun Sinn,
Gyu-Seong Choi, Hee Chul ParkID, Jong Man Kim, Honsoul Kim,Kyoung et al.Multidisciplinary
approach is associated with improved survival of hepatocellular carcinoma
patients.January 2019.PLoS ONE14(1):e0210730.
18.Hanna RF,
Miloushev VZ, Tang A, Finklestone LA, Brejt SZ, Sandhu RS, et al. Comparative
13-year meta- analysis of the
sensitivity and positive predictive value of ultrasound, CT, and MRI for
detecting hepatocellular carcinoma.
Abdom Radiol (NY). 2016;41:71–90.
19.Lee YJ,
Lee JM, Lee JS, et al. Hepatocellular carcinoma: diagnostic performance of
multidetector CT and MR imaging-a systematic review and meta-analysis.
Radiology. 2015;275:97e109.
20. Luca Di
Tommaso, Marco Spadaccini, Matteo Donadon, Nicola Personeni, Abubaker Elamin,
Alessio Aghemo,Ana Lleo. Role of liver biopsy in hepatocellular carcinoma.
World J Gastroenterol 2019 October 28; 25(40): 6041-6052.
21.Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma.Hepatology 2008;47:97–104.
22.Cabibbo G, Enea M, Attanasio M,
Bruix J, Craxi A, Camma C. A meta- analysis of survival rates of patients in randomized clinical trials
of hepatocellular carcinoma. Hepatology 2010;51:1274–1283.
23. Lei Chang, Yitao Wang, Jibo
Zhang and Tao Guo1. The best strategy for HCC patients at each BCLC stage: a
network meta-analysis of observational studies. Oncotarget, 2017, Vol. 8, (No.
12), pp: 20418-20427.
24.Marrero
JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, et al. Prognosis of
hepatocellular carcinoma:
comparison of 7 staging systems in an American cohort. Hepatology
2005;41:707–716.
25.Miller G, Schwartz LH, D'Angelica
M. The use of imaging in the diagnosis and staging of hepatobiliary malignancies. Surg Oncol Clin N Am
2007;16:343-368. Available http://www.ncbi.nlm.nih.gov/pubmed/17560517.
26.Cabibo
G,Attanasio M,BruixJ,Craxi A,Camma C. A meta-analysis of survival rates of
untreated patients in randomized clinical trials of hepatocellular carcinoma.
Hepatology (Baltimore, Md.), 01 Apr 2010, 51(4):1274-1283
27.Cooper GS, Bellamy P, Dawson NV,
et al. A prognostic model for patients with end-stage liver disease.
Gastroenterology
1997;113:12781288. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9322523.
28.Jie Chen, Yujen Tseng, Tiancheng Luo, Na Li, Lili Ma,
Shiyao Chenille . Prophylactic Endoscopic Therapy for Variceal Bleeding
in Patients with Hepatocellular Carcinoma. J
Cancer 2019; 10(14):3087-3093. doi:10.7150/jca.30434
29.
Sun DW, An L, Wei F, et al. Prognostic significance of parameters from
pretreatment (18)F-FDG PET in hepatocellular carcinoma: a meta-analysis. Abdom
Radiol (NY) 2016;41:33-41
30. Byungje Bae, Keera Kang, Sung Kyu Song, Chul-Woon Chung,
Yongkeun Park. Is partial hepatectomy a curable treatment option for
hepatocellular carcinoma accompanied by cirrhosis? A meta-analysis and cure
model analysis. Ann Hepatobiliary Pancreat Surg 2022;26:4757.
31.Senbel A, Elmahdy Y, Roshdy S, et
al. Role of Hepatic Resection for HCC in the era of Transplantation; an
Experience of Two Tertiary Egyptian Centers. Indian J Surg Oncol.
2017;8(4):514–518.
doi:10.1007/s131930170679-5.
32.Yu Huang,
Wenhao Ge, Yang Kong, Yuan Ding, et al. Preoperative Portal Vein Embolization
for Liver Resection: An updated meta-analysis. Journal of Cancer 2021, Vol. 12(6):
1770-1778.
33.OPTN/UNOS policy notice modification to hepatocellular carcinoma
(HCC) extension criteria. Available at:
https://optn.transplant.hrsa.gov/media/2411/modification-to-hcc-autoapproval-criteria_policy-notice.
34.Antonio
Facciorusso , Mohamed A. Abd El
Aziz , Nicola Tartaglia , Daryl Ramai , et al. Microwave Ablation
Versus Radiofrequency Ablation for Treatment of Hepatocellular Carcinoma:
A Meta-Analysis
of Randomized Controlled Trials. Cancers 2020, 12, 3796.
35. Francesco Izzo,
VincenzaI Granta, Roberta Grassi,Roberta Fusco, et al. Radiofrequency Ablation
And Microwave Ablation in Liver Tumors: An
Update. The Oncologist 2019;24:e990–. e1005.
36. Yun Ku Cho, Jae
Kyun Kim, Mi Young Kim, Hyunchul Rhim, et al.Systematic Review of Randomized
Trials for Hepatocellular Carcinoma Treated
with Percutaneous Ablation Therapies. Hepatology.
2009;49(2):453–9.
37. Swierz MJ,
Storman D, Riemsma RP, Wolff R, et al. Percutaneous ethanol injection for liver
metastases(Systematic Review). Cochrane
Database of Systematic Reviews 2020, Issue 2. Art. No.: CD008717.
38. Yanyan Long, Yan
Liang, Shujie Li1, Jing Guo1, et.al. Therapeutic outcome and related predictors
of
Stereotactic
body radiotherapy for small
liver-confined HCC: a systematic review and meta-analysis of
observational studies. Radiat Oncol (2021)
16:68 https://doi.org/10.1186/s13014-021-01761-1
39. Yi Yang, Zhuo-Min
Lv, Min Yan, Hong-Xin Zhang,et al. Transarterial chemoembolization combined
With radiofrequency
ablation in the treatment of hepatocellular carcinoma. April 2019.Hepatoma Rsearch 5.
40. Chao Yin, Samantha
Armstrong, Richard Shin, Xue Geng, et al. Bridging and downstaging with TACE
in early and
intermediate stage hepatocellular carcinoma: Predictors of receiving a liver
transplant. Ann
Gastroenterol Surg.2023;7:295–305.
41.Llovet J,Josep M.
Systematic review of randomized trials for unresectable hepatocellular
carcinoma:
chemoembolization improves survival.
Hepatology. 2003;37(2):429–442. doi:10.1053/jhep.2003.50047.
42. Lencioni, Riccardo*; de Baere, Thierry; Soulen, et al. Lipiodol transarterial chemoembolization for
Hepatocellular carcinoma: A systematic review
of efficacy and safety data. Hepatology 64(1):p 106-
116,7/2016-116,7/2016. July 2016.
43.Terzi
E,Golfiieri R,Piscaglia F,Giampalma E.Response rate and clinical outcome of HCC
after first and repeated
TAC performed
“on demand”. Journal of hepatology.Volume 57,Issue6,P1258-1267,12/2012.
44. Tsochatzis EA, Fatourou E, O'Beirne J, Meyer T, Burroughs AK.Transarterial chemoembolization and bland
Embolization for hepatocellular carcinoma., World J Gastroenterol. 2014 Mar 28;20(12):3069-77.
45. Maria-Aggeliki Kalogeridi, Anna Zygogianni, George Kyrgias, John Kouvaris, et al. Role of radiotherapy in the
Management of hepatocellular carcinoma: A systematic review. World J Hepatol 2015 January 27; 7(1): 101-112.
46. Park W, Lim DH, Paik SW, et al. Local radiotherapy for patients with unre- sectable hepatocellular carcinoma. Int
J Radiat Oncol Biol Phys. 2005;61(4): 1143–1150.
47. Laura A. Dawson, Cynthia Eccles , Tim Craig. Individualized image guided iso-NTCP based liver cancer SBRT.
Acta Oncologica, 2006; 45: 856864.
48. Mihir D. Shanker, Pereshin Moodaley, Wei Soon, Howard Y. Liu, et al. Stereotactic ablative radiotherapy for
Hepatocellular carcinoma: A systematic review and meta-analysis of local control, survival and toxicity outcomes.
Journal of Medical Imaging and Radiation Oncology 65 (2021) 956–968
49. Donatella C, Stefano B, Ivana R, Adelaide M. Stereotactic Body Radiation Therapy for Liver Lesions. A Single-
institution Experience. ANTICANCER RESEARCH 35:4171-4176 (2015).
50. HoonSikChoi, KiMunKang,Effectiveness of stereotactic body radiotherapy for portal vein tumor thrombosis in
patients with hepatocellular carcinoma and underlying chronic liver disease. Asia-Pac J Clin Oncol. 2021;17:209–215.
51. Soliman H, Ringash J, Jiang H, et al. Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver
metastases. J Clin Oncol 2013;31:3980-3986.
52. Yanyan Long, Yan Liang, Shujie Li, Jing Guo, Ying Wang, Yan Luo and Yongzhong Wu. Therapeutic outcome and
related predictors of stereotactic body radiotherapy for small liver-confined HCC: a systematic review
and meta-analysis of observational studies. Radiat Oncol (2021) 16:68
53. Jie Shen, Jing Yan, Sihui Zhu, Weiwei Kong, Zhengyun Zou, Juan Liu, Shuangshuang Li and Baorui Liu. The
Efficacy and Safety of Hypofractionated Radiation Therapy With Tomotherapy for Advanced or Recurrent Hepatocellular
Carcinoma. Frontiers in Oncology. May 2021 | Volume 11 | Article 559112.
54. WonIl Jang , Sunmi Jo , Ji Eun Moon , Sun HyunBae, and HeeChulPark. The Current Evidence of Intensity-
Modulated Radiotherapy for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2023,15,4914.
55. Mohamad Bassam Sonbol, MD; Irbaz Bin Riaz, MD, MS; Syed Arsalan Ahmed Naqvi, MBBS; et al. Systemic Therapy
and Sequencing Options in Advanced Hepatocellular Carcinoma A Systematic Review and Network Meta-analysis. JAMA
Oncology December 2020 Volume 6, Number 12 .
56. Bingru Xie, M.D., David H. Wang, M.D., and Stuart Jon Spechler, M.D. Sorafenib for the Treatment of Hepatocellular
Carcinoma: A Systematic Review. Dig Dis Sci. 2012 May ; 57(5): 1122–1129.
57. Bruix J, Qin S, Merle P, et al: Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib
treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389:5666, 2017.
58. Robyn Laube,Abdul-Hamid Sabih, Simone I Strasser, Lynn Lim, Maria Cigolini and Ken Liu. Palliative care in
hepatocellular carcinoma. Journal of Gastroenterology and Hepatology 36 (2021) 618–628.
59. Kumar M, Panda D. Role of supportive care for terminal stage hepatocellular carcinoma. J Clin Exp Hepatol
2014;4:S130–S139.
60. Mauro Giuffrè , Enrico Zuliani , Alessia Visintin , Paola Tarchi , Paola Martingano , et al. Predictors of
Hepatocellular Carcinoma Early Recurrence in Patients Treated with Surgical Resection or Ablation Treatment: A
Single-Center Experience. Diagnostics 2022, 12, 2517.
61. Lencioni R. New Data Supporting Modified RECIST (mRECIST) for Hepatocellular Carcinoma. Clin Cancer Res.
2013;19(6):1312–1314. doi:10.1158/1078-0432.CCR-12-3796.
- Annexes
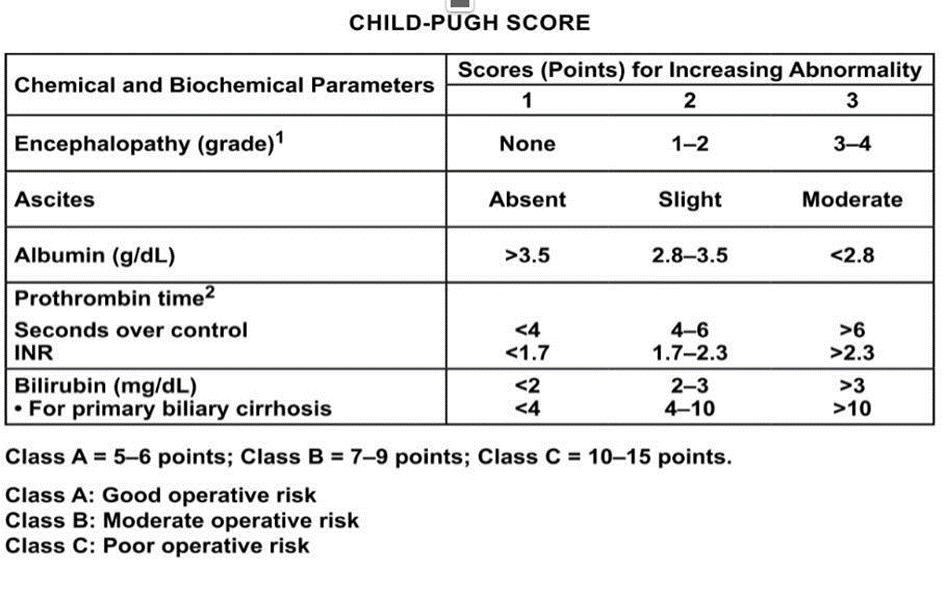
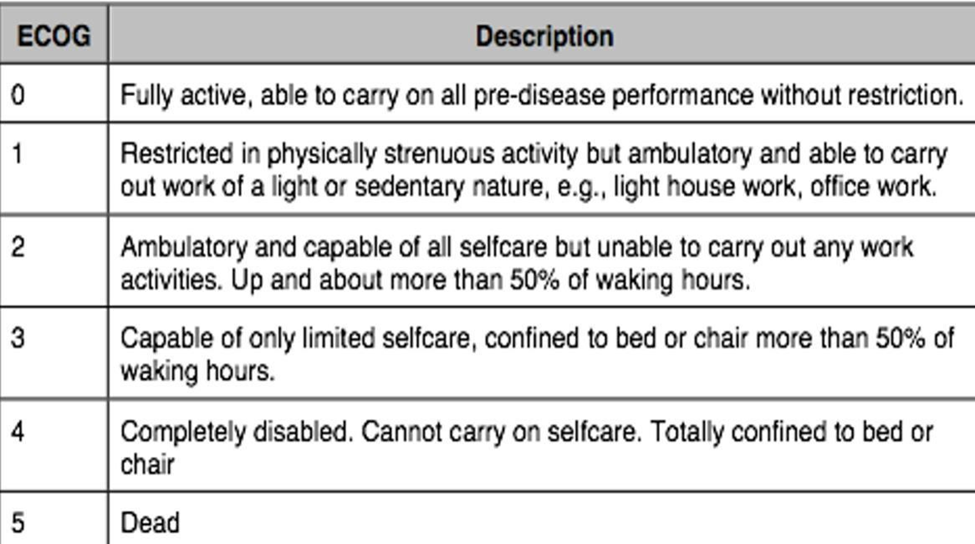
Assessment of clinical performance status BCLC staging and treatment strategy in 2022.