Acute Rhinosinusitis
- Annexes
Editorial Independence:
▪️ This guideline was developed without any external funding.
▪️ All the guideline development group members have declared that they do not have any competing interests.
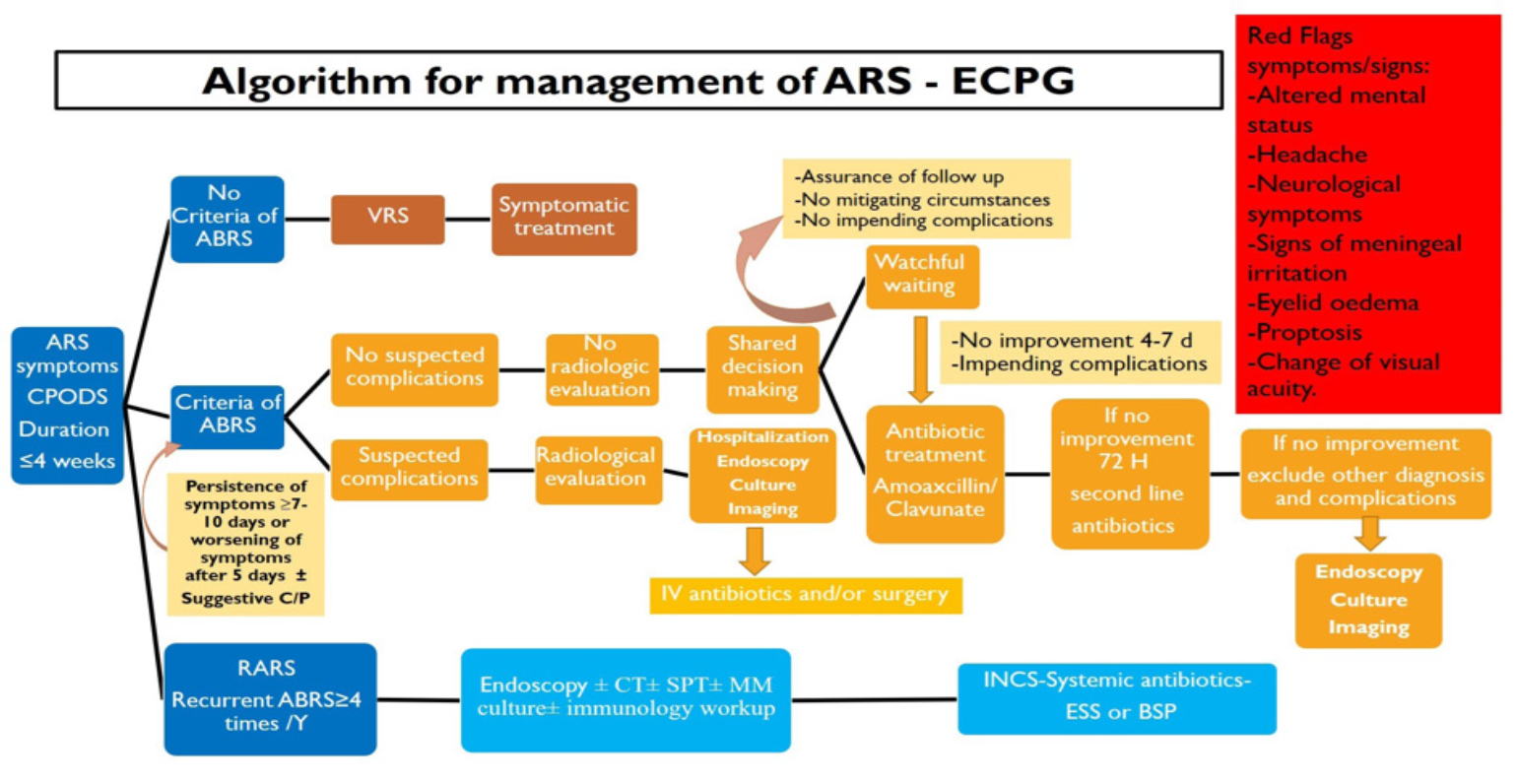
Annex 1: Guideline Flowchart
Annex 2: Tables of appraisal of selected guidelines: Currency (table 1), Content (table 2) and Quality (table 3) of the selected guidelines.
|
No |
Guideline Name |
Year of publication |
The Organization |
Age demography |
|
1. |
University of Michigan Health System guidelines on treatment for acute sinusitis UMHS |
2018 |
University of Michigan Health System(UMHS) |
Adult |
|
2. |
IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. IDSA-CPGABRS |
2012 |
Infectious Diseases Society of America |
Adult and children |
|
3. |
NICE |
2017 |
National Health System NHS England |
Adult and children |
|
4. |
Canadian guidelines for acute bacterial rhinosinusitis Canadian medical societies CMC |
2014 |
Canadian medical societies (Association of Medical Microbiology and Infectious Disease Canada, Canadian Society of Allergy and Clinical Immunology, Canadian Society of Otolaryngology—Head and Neck Surgery, Canadian Association of Emergency Physicians, and the Family Physicians Airways Group of Canada) |
Adult |
|
5. |
Standards of Care Committee of the BSACI BSACI |
2007 |
British Society for Allergy and Clinical Immunology. Standards of Care Committee of the BSACI |
Adult |
|
6. |
American Academy of Pediatrics clinical practice guideline EAACI |
2013 |
American Academy of Pediatrics |
Children |
|
7. |
EPOS |
Published 2012 Updated 2020 |
Task force commissioned by the EAACI |
Adult and children |
|
8. |
American Academy of Otolaryngology – Head and Neck Surgery AAO-HNSF |
Published 2007 Updated 2015 |
American Academy of Otolaryngology – Head and Neck Surgery Foundation |
Adult |
|
9. |
AAAAI/ACAAI practice parameter of ARS |
2014 |
American Academy of Allergy, Asthma & Immunology (AAAAI)/The American College of Allergy, Asthma & Immunology (ACAAI) |
Adult |
Table 1
|
Criteria |
Guideline A UMHS |
Guideline B NICE |
Guideline C EPOS |
Guideline D AAO-HNSF |
|
Credibility |
9 |
9 |
9 |
9 |
|
Observability |
8 |
7 |
8 |
8 |
|
Relevance |
7 |
7 |
7 |
8 |
|
Relative advantage |
7 |
7 |
7 |
8 |
|
Easy to install and understand |
7 |
7 |
7 |
9 |
|
Compatibility |
7 |
7 |
7 |
8 |
|
Testability |
7 |
7 |
7 |
8 |
|
Total score |
52 |
51 |
52 |
58 |
Table 2
|
Domain |
Guideline A UMHS |
Guideline B NICE |
Guideline C EPOS |
Guideline D AAO-HNSF |
|
B |
A |
B |
A |
|
2. Conflict of interest |
B |
NR |
NR |
A |
|
3. Development group |
B |
B |
B |
B |
|
4. Systematic review |
A |
A |
A |
A |
|
5. Grading of evidence |
A |
NR |
A |
A |
|
6. Recommendations |
B |
C |
B |
A |
|
7. External review |
B |
A |
A |
A |
|
8. Updating |
A |
A |
A |
A |
Table 3
Annex 3: The risks and benefits of added and/or modified statements
|
The statement to be adapted: Action |
Benefits |
Risk/Harm |
|
|
Symptoms must include: 1–Mucopurulent nasal drainage/discharge (anterior/ posterior) and 2–Nasal blockage/obstruction/congestion or facial pain/ pressure or both Symptoms may include smell affection and headache in adults and cough in children. All can be included in CPODS C: Congestion P: Pain O: Obstruction D: Drainage/Discharge S: Smell affection. |
Easy to remember letters |
No |
|
|
A thorough physical examination that includes inspection, palpation of the maxillary and frontal sinus, as well as anterior rhinoscopy (evidence of inflammation, mucosal oedema, and discharge). |
-Allow physician to assess the patient without radiological diagnostics. -Little skills and Equipment's are needed |
More time needed for settlement of appointments |
|
|
ESR and CRP are inflammatory markers found to be elevated during ARS, but they are not routinely used for diagnosis because of their limited specificity. They may have some role in COVID-19 related symptoms in Chinese guidelines for COVID-19: CRP test together with other clinical parameters for initial evaluation and follow-up of coronavirus infection. Cut-off for CRP: 40–50 mg/L (4). |
Easy and rapid laboratory investigation that have some values to diagnose covid patients |
Low evidence and it can be elevated with other causes |
|
|
Consider initial watchful waiting in uncomplicated cases, with institution of antibiotic therapy if no improvement after 4-7 days or worsening at any time, or for mitigating circumstances with drug resistance e.g., including severe symptoms, immunocompromised state, concern for impending complications, suspected odontogenic source, prior antibiotics (1 month), prior hospitalization (5 days) and comorbidities. Watchful waiting should be offered only when there is assurance of follow-up, such that antibiotic therapy is started if the patient’s condition fails |
Decrease the use of antibiotics with limited duration of waiting policy and criteria of assurance of follow up |
The patient need to improve rapidly and the high burden over doctors |
|
|
An alternative management strategy is recommended if symptoms worsen after 48–72 hours of initial empiric antimicrobial therapy or fail to improve despite 3–5 days of initial empiric antimicrobial therapy If symptoms persist or worsen despite 72 hours of treatment with a second-line regimen, Additional investigations (such as sinus puncture or acquisition of cultures of the middle meatus, and CT or MRI studies) should be initiated. |
Provide a systematic and algorithm-based approach to antimicrobial therapy of patients failing initial therapy. |
The potential for adding more selection pressure for resistance due to ‘‘antimicrobial surfing’’ and adding adverse effects without antimicrobial benefit. |
|
|
INCS can be used according to the doctor judgment as monotherapy in mild to moderate ARS or as adjuvant to antibiotic therapy in severe cases of ARS.
|
Doctors' judgment can allow building the balance between benefits and harms |
Cost and local side effects |
|
|
-First time non-responders can be based on lack of clinical improvement following treatment within 5 days in adults and 3 days in children. -B-lactamase producing penicillin resistance H.Influenzae in > 30 % and S.pneumoniae. -Use second line antimicrobial agents. -Second time non responders who fail to improve with second line antibiotic therapy should be evaluated for other diagnosis or considered for sinus aspiration or endoscopically guided middle meatus culture and sensitivity. |
Decrease the time that the patient to be left without follow up and improvement |
Increase the risk of bacterial resistance |
|
|
At least 4 attacks of ABRS/year are a required criterion -Nasal endoscopy and/or CT imaging are an option during at least one episode of suspected RARS to appropriately confirm and diagnose RARS, and distinguish it from other diagnoses such as allergy exacerbation or primary headache syndromes. -Consider immunologic testing, allergic testing, and bacterial culture in patients with concern for RARS -Option for use of INCS spray for sinonasal symptoms during acute exacerbations of RARS. -Follow other ABRS management options -Endoscopic sinus surgery (ESS) is recommended for patients with RARS. |
Baloon sinusoplasty was excluded as there are limited evidence for improvement |
High cost with limited benefit |
|